

47114-G6
Heterogeneous Reactivity of Trace Elements in Combustion Flue Gases
The binding mechanism of Hg on noble metals, Pd binary alloys and overlays and their surface reactivity has been investigated by examining their electronic structure. In this manner DFT calculations have been carried out to examine the Hg binding on bridge, top and hollow sites of Pd(111), M(111) (M=Au, Ag, Cu), Pd-M(111) binary alloys and Pd/M(111) overlays. Differences in the binding energies on hcp and fcc hollow sites are found to be negligible and no stable geometry was found on bridge and top adsorption sites. Binding energies of Hg on four noble metals are found in the following order: Ebind(Pd) > Ebind(Cu) > Ebind(Ag) > Ebind(Au).
|
 In Pd-M alloys,
increasing the percentage of Au, Ag and Cu in Pd binary alloys causes Hg
binding to be weaker. Enhanced Hg binding can be obtained when Hg lies closer to the surface Pd atoms rather than the M atoms. The addition of 25% Ag or Au in Pd binary alloys can
either improve Hg binding, or decrease the binding energy of Hg depending on
the adsorption site. This behavior demonstrates the sensitivity of Hg binding
to the position of the Pd and M atoms surrounding the adsorption sites.
Specifically, M atoms improve the Hg reactivity of surface Pd atoms, when they
are located in subsurface layers of the alloy. †Thus, the next step is to examine Hg adsorption
on overlays of Pd on M(111) surfaces. In all overlays,
Pd overlay substrates might increase the binding energy of Hg in comparison to M(111) surfaces. In particular, Pd/Au(111)
and Pd/Ag(111) overlaying results in increased Hg binding energies compared to
those of Pd(111) surfaces.† In both cases
binding energies of Hg are up to 0.1-0.2 eV larger
than on the pure Pd(111) surface and reach a maximum value on the surface of
two Pd overlays. Larger binding energies on the Pd/Au(111)
and Pd/Ag(111) overlays are the result of the lattice expansion of Pd
substrates (geometric effect) which results in inducing an electronic effect of
the underlying host.†
In Pd-M alloys,
increasing the percentage of Au, Ag and Cu in Pd binary alloys causes Hg
binding to be weaker. Enhanced Hg binding can be obtained when Hg lies closer to the surface Pd atoms rather than the M atoms. The addition of 25% Ag or Au in Pd binary alloys can
either improve Hg binding, or decrease the binding energy of Hg depending on
the adsorption site. This behavior demonstrates the sensitivity of Hg binding
to the position of the Pd and M atoms surrounding the adsorption sites.
Specifically, M atoms improve the Hg reactivity of surface Pd atoms, when they
are located in subsurface layers of the alloy. †Thus, the next step is to examine Hg adsorption
on overlays of Pd on M(111) surfaces. In all overlays,
Pd overlay substrates might increase the binding energy of Hg in comparison to M(111) surfaces. In particular, Pd/Au(111)
and Pd/Ag(111) overlaying results in increased Hg binding energies compared to
those of Pd(111) surfaces.† In both cases
binding energies of Hg are up to 0.1-0.2 eV larger
than on the pure Pd(111) surface and reach a maximum value on the surface of
two Pd overlays. Larger binding energies on the Pd/Au(111)
and Pd/Ag(111) overlays are the result of the lattice expansion of Pd
substrates (geometric effect) which results in inducing an electronic effect of
the underlying host.† The reactivity of a transition metal depends on the position of the d-band center relative to the Fermi level, d-band width and the occupancy of the d-bands. The d-band center for the surface (εd) metal atoms were calculated by taking the first moment of the normalized projected density of states up to the Fermi level.1
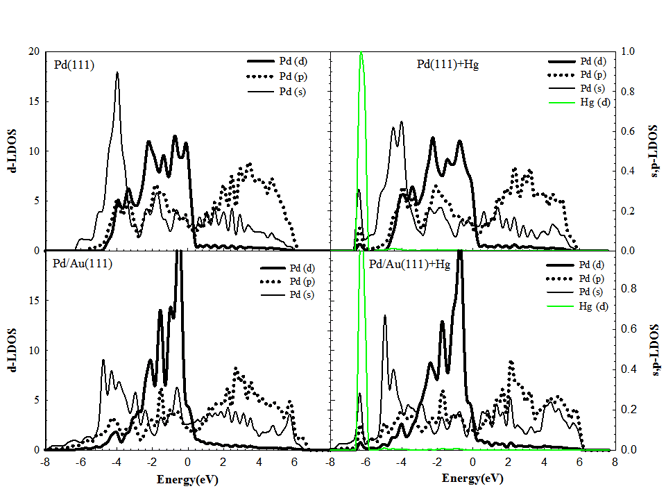
As the d-band center shifts up in energy, enhanced Hg interaction is been observed. Specifically, the LDOS of the Pd/Au(111) surface presented in Figure 1, exhibit the enhanced binding compared to that of pure Pd(111). It is also clear from Figure 1 that the d-band width of Pd on clean Pd/Au(111) surface becomes narrower due to the hybridization of the d-states of the surface atoms with the second layer atoms. Also, the d-band center of the surface atoms is found to be higher in energy than that of the clean Pd(111) surface, which improves the reactivity. In all of the surfaces examined, the d-band of Hg overlaps strongly with the s- and p-band of Pd at ~ 7eV. It has been determined that the s- and p-band of Pd exhibit an increase in their DOS after Hg binding.
The position of M atoms located in both surface and subsurface layers may enhance and reduce the reactivity of the surface, respectively.† As Pd is deposited on the top of another metal having a larger lattice spacing than Pd, the overlaying substrate assumes the lattice constant of the underlying metal and the d-states of the surface Pd atoms shifts up in energy, which leads to a high surface reactivity.
References
1Hammer, B.; Nielsen, O.H.; NÝrskov, J.K.† Catal. Lett. 1997, 46, 31.