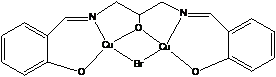44483-GB3
An Electrochemical Investigation of Small Molecule Mimics of Multicopper Oxidases
The undergraduates in my laboratory have continued to use electrochemistry to study electron-transfer reactions relevant to further our understanding of fuel cell and solar cell processes. Our work has recently addressed two areas: electron-transfer mechanisms of multicopper complexes and the use of scanning electrochemical microscopy to study dye sensitized solar cells.
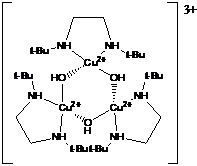
In the first year of support, we utilized literature preparations to synthesize several multicopper(II) complexes containing oxo-bridges, 1, or Schiff base ligands, 2.1,2 In each case, the complexes exhibited significant electrode adsorption problems that complicated a rigorous analysis of the electrochemical data. We have since identified appropriate solvent/supporting electrolyte conditions that minimize adsorption of electrochemically generated Cu(I). Future work will focus on conducting systematic electrochemical studies of these complexes in hopes to understand the relationship between electron-transfer rates and structural changes upon reduction.
1 |
2 |
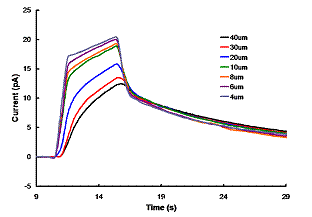
Figure 1 SECM time of flight current transients. An ultramicroelectrode is positioned at a fixed distance above a photosensitized thin film. Upon illumination, the thin film produces oxidized species detected by the electrode. |
The second aspect of this research involves utilizing scanning electrochemical microscopy (SECM) to study electron transfer between a photosensitized titanium dioxide thin film and an electrolyte solution containing a redox mediator. We set out to demonstrate the feasibility of using time-of-flight electrochemical measurements to probe mass- and electron transport between an illuminated nanocrystalline titanium dioxide thin film and an electrode bathed in an electrolyte containing a redox mediator. Preliminary results, which were presented by an undergraduate research student at the national ACS meeting in New Orleans (Spring 2008) demonstrate that SECM is sensitive to photoinduced redox processes at the solid/liquid interface, and through theoretical simulations, one can extract pertinent kinetic data about the electron transfer process. The data (Figure 1) show that the electron-transfer process is a surface event and that two processes, presumably diffusion through the nanoporous thin film and diffusion through the electrolyte medium, are influencing the shape of the current-time transient.
Finally, two projects related to the proposed work resulted in publications. In the first case, we have demonstrated our ability to use SECM to study electron-transfer events in viscous solvents. Numerical simulations of approach curve data obtained in a deep eutectic solvent support our assertion that mass transport due to movement of the ultramicroelectrode must be considered when analyzing the current-distance data. We proposed a set of working curves that describe the shape of the approach curve using a single parameter, the Peclet number, which is the ratio of convective to diffusive mass transport. In the second paper, we analyzed the electrochemical oxidation of di-tertbutyl nitroxide, DTBN, using SECM. Traditional electrochemical methods have been used to study DTBN,3 however the results were complicated by uncertainty imposed by solution resistance and mass-transport limitations of the methods. We demonstrated that SECM could be used to better understand qualitative and quantitative aspects of the electron transfer mechanism.
In addition to the scientific contributions which have developed from this research, PRF funding has allowed three undergraduate students to present at national ACS conferences and four additional students to attend local undergraduate research symposia. Of the eight students who participated in this research, 7 are African American and 3 are women. Several of these students have since graduated: one is currently in a biology graduate program; one is in medical school; and one is currently finishing courses to become a science teacher in the Chicago Public School system.
1 Mirica, L. M.; Stack, T. D. P. Inorg. Chem., 2005, 44, 2131.
2 Mukherjee, A.; Rudra, I.; Naik, S. G.; Ramasesha, S.; Nethaji, M.; Chakravarty, A. R. Inorg. Chem., 2003, 42, 5660.
3. Kishioka, S.-y.; Yamada, A. Electrochim. Acta 2005, 51, 462.