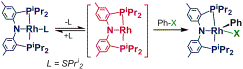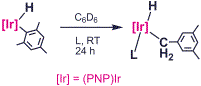44904-AC3
Rigid Pincer Ligands: New Prospects in Hypercoordinate Main Group Chemistry
Fafard, C. M.; Chen, C.-H.; Foxman, B. M.; Ozerov, O. V. "Covalent Palladium-Zinc Bonds and Their Reactivity", Chem. Commun. 2007, 4465.
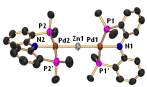
Photolysis of (PNP)Pd-Et in the
presence of Et2Zn resulted in the formation of (PNP)Pd-Zn-Pd(PNP),
containing a linear Pd-Zn-Pd linkage with approximately square-planar
environments about the Pd centers. This
is the first example of a compound with a covalent Pd-Zn bond. It displays unusual reactivity in the
reaction with MeI: the kinetic products are (PNP)Pd-I and Me2Zn(!).
Gatard, S.; Guo,
C.; Foxman, B. M.; Ozerov, O. V. "Thioether,
Dinitrogen, and Olefin Complexes of (PNP)Rh: Kinetics and Thermodynamics of
Exchange and Oxidative Addition Reactions", Organometallics 2007, 26, 6066.
(PNP)Rh-L compounds where L = R2S
or bulky olefin are convenient synthons for the transient (PNP)Rh. We determined that (PNP)Rh-SPri2
(and likely other (PNP)Rh-L) reacts with PhBr via reversible dissociation of
SPri2 prior to addition of PhBr. In the same study, we determined the
relative affinity of (PNP)Rh for a series of L ligands. Here are some of the findings: a) steric
effects are dominant in the binding of dialkyl sulfides; b) SPri2
binds more strongly than Me3CCH=CH2, more weakly than N2;
c) consistently with a dissociative mechanism, the rate of the PhBr OA
reactions to (PNP)Rh-L decreases with the increased strength of binding of L;
d) (PNP)Rh(SPh2) is isoergic with its C-S OA isomer
(PNP)Rh(Ph)(SPh): a 1:1 equilibrium is rapidly established at 70 °C.
Zhu, Y.; Fan,
L.; Chen, C.-H.; Finnell, S. R.; Foxman, B. M.; Ozerov, O. V. "C-H Oxidative Addition to a (PNP)Ir Center
and Ligand-Induced Reversal of Benzyl/Aryl Selectivity", Organometallics
2007, 26, 6701.
Complex (PNP)Ir(H)(Mes) is an
attractive synthon for (PNP)Ir. For
instance, it activates benzene or toluene solvent via rate-limiting reductive
elimination of mesitylene at a reasonable rate (t1/2 < 30 min at
60 °C). With pyridine, instead,
isomerization to the benzylic isomer of mesityl was observed. The drive for isomerization is primarily
owing to steric factors: isomerization of mesityl to a less sterically imposing
3,5-dimethylbenzyl by itself is not favorable, but is compensated by the
stronger binding of pyridine to the latter.
Additional conclusions from this study are that 1) the aryl-to-benzylic
isomerization in (PNP)Ir(H)(Mes) is kinetically readily accessible and is
faster than loss of mesitylene and 2) reductive elimination of mesitylene does
not proceed from the 6-coordinate Ir, but only from the five-coordinate Ir
(this echoes other studies on RE from d6 complexes).
Bontemps, S.;
Bouhadir, G.; Gu, W.; Mercy, M.; Chen, C.-H.; Foxman, B. M.; Maron, L.; Ozerov,
O. V.; Bourissou D. "Metallaboratranes
Derived from a Triphosphanyl-Borane: Intrinsic C3 Symmetry Supported
by a Z-Type Ligand", Angew. Chem. Int. Ed. 2008, 47, 1481.
Our initial synthetic attempts at
the preparation of PBP pincers led instead to the C3-symmetric BP3
ligand. At that time, we discovered
that BP3 was also being investigated by the Bourissou group at Univ.
Paul Sabatier (Toulouse, France). We
thought it most scientifically productive to combine efforts in a joint
manuscript describing the synthesis, structure, and computational analysis of
BP3 complexes of Pt and Au.
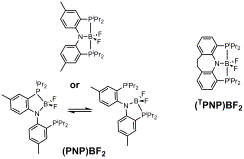
Pursuit of five-coordinate boron. We were able to synthesize (PNP)BF2
which displays apparent C2v symmetry in its NMR spectra down to –80 °C. Strong coupling was observed among the two 31P,
the 11B, and the two 19F nuclei, consistent with a
five-coordinate environment at boron. However,
an X-ray study revealed a solid-state structure in which boron is
four-coordinate, with one phosphine arm of the PNP ligand being “off”. It is possible that two degenerate
four-coordinate structures are rapidly interconverting in solution with low
activation barrier. Our next step is to
investigate analogous (TPNP)BF2 complexes with the “tied”
PNP ligand. The “tied” PNP restricts
the conformational freedom of the phosphine arms and may serve better to
enforce pentacoordination at boron.