47094-AC1
De Novo Synthesis of Rare/Unnatural Sugars from Achiral Materials
The O'Doherty Group Research Summary:
Overview: My research group is interested in developing new methods for the de novo synthesis of natural and unnatural sugars. Our goal is to use asymmetric transition metal catalysis to create all the stereochemistry of the monosaccharides and oligosaccharides, in addition to using transition metal catalysis to control the stereochemistry of the glycosidic bond. We have already had great success in the preparation of common and uncommon monosaccharides, and more recently, have had success in extending this methodology toward the preparation of di- and trisaccharides (vide infra).
De Novo Synthesis of Monosaccharides: Our approach to produce various hexoses relies on an oxidative rearrangement of furfuryl alcohols to pyranones (Achmatowicz reaction). These furfuryl alcohols were produced via asymmetric catalysis (Sharpless oxidations/Noyori reductions). We have succeeded in developing a short route that is flexible enough for the synthesis of five of the eight possible diastereomeric hexoses and both deoxy- and 4- and 6-substituted aminosugars as either enantiomer.
De Novo Synthesis of Natural and Unusual Oligosaccharides: Our de novo approach has also been applied to oligosaccharides. A long-term theme to this research is the development of highly stereoselective glycosylation and post glycosylation transformations that can be used in diverse complex oligosaccharide settings (i.e., to be as reliable and predictable as the carbohydrate protecting group chemistry it replaces). The key to the success of this approach is the development of a mild palladium catalyzed glycosylation in combination with the discovery of a highly enantioselective approach to pyranones from acylfurans via Noyori chemistry. This approach has been expanded to a cyclitol installation reaction. This reaction has great potential for preparing various D- and L-sugars because the starting 6-t-butoxycarboxy-2H-pyran-3(6H)-ones (5.1, 5.2, 5.5 and 5.6) can easily be prepared from optically pure furfuryl alcohols (either (R) or (S) form) by a two-step procedure.
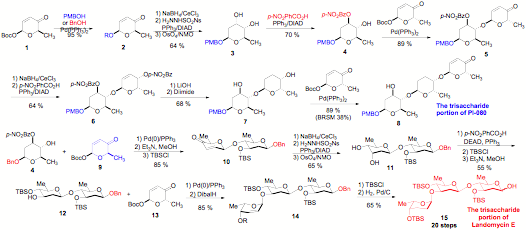
De novo synthesis of natural product trisaccharides: In fact, we have successfully applied this approach to several natural product oligosaccharides, but we feel the approach is best demonstrated in our recently reported approach to the trisaccaride portion of PI-080/Vineomycin B2 and Landomycin E (Scheme 1). Key to the success of this approach was the discovery of a highly regioselective Mitsunobu like inversion of the axial alcohol of a cis-1,2-diol (3 to 4). This transformation in combination with a highly diastereoselective dihydroxylation reaction becomes a nice practical solution to the problem of 1,2-trans-diequatorial addition to a cyclohexene. To our surprise, we found significant antitumor activity is associated with the trisaccharide portion of PI-080/Vineomycin B2
Scheme 1: Synthesis of the trisaccharide portion of Landomycin and PI-080/Vineomycin B2
Significance to Future Studies: We
have shown that a de novo asymmetric approach to carbohydrates (mono- to
oligosaccharides) allows for an easy entry to sufficient quantities complex
molecules as well as various analogues for further studies. We believe that
these de novo approaches will enable medicinal chemists to more easily perform
SAR studies on complex carbohydrate structures. In fact, we have already
started to demonstrate this utility. These new approaches to carbohydrates
provide a practical access to unique complex molecules that are not readily
accessible by traditional carbohydrate routes.