

46022-GB4
Influence of Ring Size and Substituents on the Cyclopropylcarbinyl Radical Fragmenttion
During this past year we have explored the thermokinetics of fragmentation of 1- bicyclo[n.1.0]alkylmethyl radicals (e.g., 1) and related systems. Our initial efforts were focused on 1, the prototype system (Scheme 1), with more recent investigations aimed at benzofused derivatives. We are pleased to report that the investigation of the five-membered ring (1, n=1) has resulted in a publication. The prominence of this particular system in many earlier cyclopropylcarbinyl radical explorations justified it being submitted as a standalone study. What's more, this paper includes five undergraduate research co-authors.
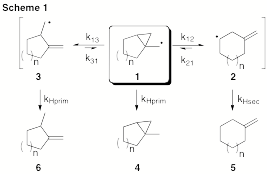
Our studies of 1-bicyclo[5.1.0]octanylmethyl radical (1, n=3) and 1- bicyclo[6.1.0]nonylmethyl radical (1, n=4) are also complete and a manuscript is presently in preparation. The rate expressions for the seven-membered ring (n=3) were determined to be log(k12/(s-1)) = 13.57 – 8.96/Θ and log(k13/(s-1)) = 13.23 – 7.55/Θ. The rate expressions for the eight-membered ring (n=4) were determined to be log(k12/(s-1)) = 12.22 – 6.49/Θ and log(k13/(s-1 )) = 12.54 – 6.70/Θ. These analyses were performed over a temperature range of –78 to 56 °C.
We initially planned to disseminate the results for the two medium-ring substrates (n = 3 and 4) together, and then report larger ring sizes (n = 6, 8, 11) in a separate submission. These ring sizes were chosen based on the commercially availability and cost of the corresponding cycloalkanone starting materials. However, the larger rings are proving to be a point of consternation in several respects.
First, the twelve- and fifteen-membered rings have presented solubility issues for some steps along the synthetic sequence. Also, as anticipated for the larger ring sizes (n > 4), the possibility of producing E and Z isomers during the preparation of the radical precursors was realized. This is shown specifically for the cyclodecyl system in Scheme 2. The geometric isomers were also observed for the cyclododecyl and cyclopentadecyl systems. This would ultimately lead to stereoisomers of the cyclopropylcarbinyl radicals (1) entering into the reaction manifold. It should be noted, however, that the ring-opened hydrocarbon products clear this stereochemical feature, irrespective of their origins (E or Z), permitting an analysis comparable to the smaller ring sizes. The resulting kinetic analysis would then be a composite of the rates of the individual E and Z isomers. Additionally, for the larger rings, isomerization of the α,β- unsaturated ester to the β,γ-unsaturated ester (e.g., 7 → 8) could not be effected to any satisfying degree through a variety of methods.
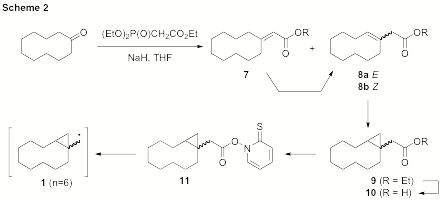
Despite these challenges, we have recently achieved preparation of the ten-membered ring cyclopropyl acid (10, R = H). This has been accomplished by performing the saponification directly on the mixture of three alkene isomers (7, 8a, 8b) obtained from the Wadsworth- Emmons reaction to provide the unsaturated acid. Interestingly, the saponification provided only two isomers, the α,β-unsaturated acid and only one of the β,γ-unsaturated acids. It is presently unclear whether this is the E or Z isomer. Nonetheless, we have found that these positional isomers could be separated by radial chromatography (column chromatography proved ineffective). Multiple chromatographic applications have been required to build up adequate material.
Cyclopropanation of the pure β,γ-unsaturated acid could not be moved to completion thereby complicating the purification as the unreacted alkene co-elutes with the cyclopropanated material. We have circumvented this problem by subjecting the crude mixture to epoxidation conditions (mCPBA, CH2Cl2), which consumes the offending alkene and enables chromatographic separation. Preparation of the radical precursor is currently underway. With the difficulties encountered for the larger rings in mind, it seems prudent to focus our efforts on completing the ten-membered ring substrate and including it with the seven- and eightmembered ring data in one manuscript.
Our investigations have more recently included the exploration of benzofused derivatives (Figure 1). As expected, we have found that radical 13 fragments exceptionally fast (k > 10 9s-1). The realized stability of benzylic radical 17 contributes significantly to this rate-enhancement (Scheme 3). When tri-n-butylstannane is used as the reducing agent the ring expansion product 18 is produced exclusively. The use of the fast trapping agent PhSeH will be required to accurately time the fragmentation of this process as the use of PhSH has been ineffective.
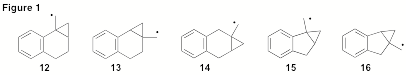
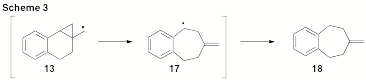
Considering the unusually fast unimolecular fragmentation rate of ring-expansion of the prototype five-membered ring (1, n = 1, k12 = 8.4 x 10 8s-1at 298 K), it will be interesting to observe the degree to which the fragmentation rate of 16 is accelerated due to the benefits of benzylic stabilization. Presently, and analogous to 13, we have identified the ring-expansion pathway as the exclusive outcome when subjected to tri-n-butylstannane. The use of PhSH has currently failed to trap any of the unopened cyclopropane derivative (cf., 4). We are currently preparing more the radical precursors so as to have enough material to complete the study.