42806-B3
Compounds of Bidentate Phosphines with Metallocene Backbones: Electrochemistry and Catalysis
Undergraduates in my lab have continued to investigate the properties of bidentate phosphines that have metallocene backbones. To date our work has focused on the synthesis, electrochemistry and structural analysis of compounds containing either
1,1'-bis(diphenylphosphino)metallocene (metallocene = ferrocene (dppf), ruthenocene (dppr) or osmocene (dppo)) or 1,1'-bis(dialkylphosphino)ferrocene (alkyl = i-propyl (dippf), cyclohexyl (dcpf) or t-butyl (dtbpf)).
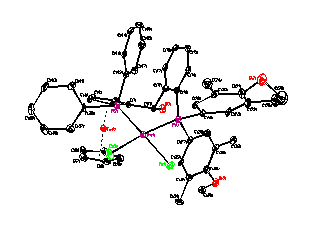
In the past year, we have completed an investigation of the asymmetric compound 1-diphenylphsophino-1'-ditertbutylphosphinoferrocene (dppdtbpf). The oxidation of dppdtbpf is chemically reversible. Upon complexation, the oxidation of dppdtbpf is typically chemically and electrochemically reversible. A series of phosphine sulfide, phosphine selenide and mixed phosphine sulfide/selenide compounds of dppdtbpf were prepared and characterized (Figure 1). The electrochemistry of these complexes is complicated if there is at least one selenium present.
| |
Figure 1. X-ray structures of C30H36FeP2SSe.
We have
also been investigating the synthesis, electrochemistry and reactivity of a
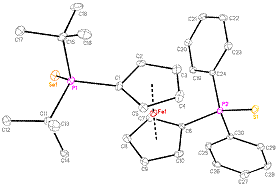
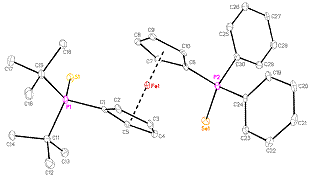
series of CpRu(P-P)H complexes (P-P = dppf, dppr, dppo, dippf or dppc). The reaction we have been examining is the
transfer of the hydride ligand from the ruthenium center to an iminium cations
(Figure 2). During this time we
developed a collaboration with Jack Norton at
Figure 2. Hydride transfer
reaction. -CF3 group, the dtbpf gave the most efficient
catalyst. Additional catalytic studies
are currently underway.
Finally, we
have continued or investigation of commercially available chiral, bidentate
phosphines with metallocene backbones.
We have examined the electrochemistry and complexation of a series of
Taniaphos and Walphos ligands (Figure 3). The electrochemistry of the ligands displays
multiple oxidation waves that are not greatly influenced by R or R'. Upon complexation, the electrochemistry
typically
Taniaphos Walphos Figure 3. Taniaphos and Walphos
ligands. simplifies to a single, chemically and electrochemically
reversible wave. The structures of three
new compounds were reported and the bite angles for Taniaphos and Walphos were
examined (Figure 4).
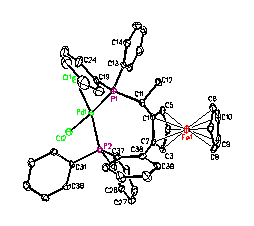
Figure 4. X-ray structures of
[(P-P)PdCl2] (P-P = Taniaphos or Walphos).