

43014-AC4
Coupled Conformational Equilibria in Peptide-Dendron Conjugates
 Peptide-guided self-assembly of
synthetic systems is emerging as a particularly powerful strategy to create
complex nanostructures from relatively simple building blocks. Such peptide-based assemblers promise
a versatile strategy to prepare functional nanostructures. The rules for
designing b-sheet forming peptides are increasingly
understood; however, it is difficult to modulate higher levels of
superstructure (i.e. fibrils versus nanotubes). Consequently, the utility of these materials are generally limited
by their tendency to undergo uncontrolled assembly into insoluble fibrils.
Previously, we observed that peptide-dendron hybrids based on an intrinsically
α-helix alanine-rich sequence underwent an α-helix to β-sheet
conformational transition going from TFE to water. The interdendron spacing of
two dendron modified alanine residues (Ad), displaying terminal
tetraethylene glycol chains to impart water solubility, was varied from i,
i+4 to i, i+10.
Circular dichroism (CD) and infrared spectroscopy studies revealed that
the i, i+6 and i, i+10 PDHs adopted a b-sheet secondary structure in PBS buffer that further
assembled into fibrils, as evidenced by atomic force microscopy (AFM). This
transition was attributed to an intermolecular hydrophobic association of the
dendritic side chains that is optimal for these dendron spacings in water.
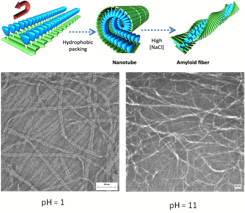
Transmission electron microscopy (TEM) analysis of the i, i+10 peptide-dendron (PDH) in pure water revealed the formation of
monodisperse nanotubular assemblies with diameters of ca. 6 nm and lengths of several micrometers. The
nanotube interconverts with an amyloid-like fibrillar structure with changes in
salt concentration or pH. The assemblies bind and release hydrophobic molecules
such as Nile Red as a function of pH, suggesting potential applications in drug
or gene delivery.
Peptide-guided self-assembly of
synthetic systems is emerging as a particularly powerful strategy to create
complex nanostructures from relatively simple building blocks. Such peptide-based assemblers promise
a versatile strategy to prepare functional nanostructures. The rules for
designing b-sheet forming peptides are increasingly
understood; however, it is difficult to modulate higher levels of
superstructure (i.e. fibrils versus nanotubes). Consequently, the utility of these materials are generally limited
by their tendency to undergo uncontrolled assembly into insoluble fibrils.
Previously, we observed that peptide-dendron hybrids based on an intrinsically
α-helix alanine-rich sequence underwent an α-helix to β-sheet
conformational transition going from TFE to water. The interdendron spacing of
two dendron modified alanine residues (Ad), displaying terminal
tetraethylene glycol chains to impart water solubility, was varied from i,
i+4 to i, i+10.
Circular dichroism (CD) and infrared spectroscopy studies revealed that
the i, i+6 and i, i+10 PDHs adopted a b-sheet secondary structure in PBS buffer that further
assembled into fibrils, as evidenced by atomic force microscopy (AFM). This
transition was attributed to an intermolecular hydrophobic association of the
dendritic side chains that is optimal for these dendron spacings in water.
Transmission electron microscopy (TEM) analysis of the i, i+10 peptide-dendron (PDH) in pure water revealed the formation of
monodisperse nanotubular assemblies with diameters of ca. 6 nm and lengths of several micrometers. The
nanotube interconverts with an amyloid-like fibrillar structure with changes in
salt concentration or pH. The assemblies bind and release hydrophobic molecules
such as Nile Red as a function of pH, suggesting potential applications in drug
or gene delivery.