46923-AC5
Determining the Effect of Local Structure on Acidity in High Surface Area Oxides
The overall goal of this research is to understand how the local variations in the structure of layered niobates affects the surface properties of the material. In this particular study our focus is on the surface acid sites present in the nanosheet versions of these materials that are produced upon exfoliation. To examine these local changes, 93Nb solid-state NMR has been used to measure both the electric field gradient (EFG) and the chemical shift anisotropy (CSA) at the niobium sites. As both effects are sensitive to the local bonding of the niobium, they can serve as guides to small changes in local structure and symmetry.
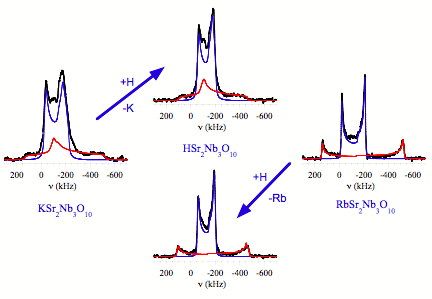
Building from our previous work[1] on the triple-perovskite layered Dion-Jacobson phase, KCa2Nb3O10, it was clear that there are two different NMR environments in these layered materials: a niobium site at the interface between layers and a niobium environment in the center of the layer. To understand the effects of the alkaline earth cation on the local structure, strontium-containing Dion-Jacobson phases were studied with potassium or rubidium as the alkali cation. While the interface and interior niobium sites in the calcium containing forms had quadrupolar coupling (CQ) values for the EFG typical of pervoskite niobates (20 – 30 MHz), the two different niobium environments in the strontium-containing materials had couplings that ranged from 45 – 93 MHz. The large values indicate that a substantial amount of strain must be present at the niobium sites due in part to the increased size of the strontium atom and is consistent with the observed increase in the lattice parameters of the niobate layers. Initial calculations of the EFG in the RbSr2Nb3O10 sample using periodic density functional theory methods point to the source of the strain coming from a possible tilting of the niobate octahedra as has been suggested from Raman spectroscopy.[2]
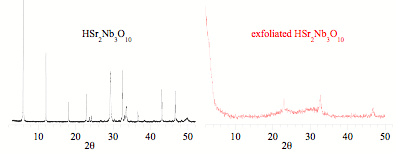
To understand the effect of cation replacement, the pre-exfoliated acid-exchanged forms of the KSr2Nb3O10 and RbSr2Nb3O10 samples were also examined using solid-state NMR methods. From x-ray powder diffraction studies, the acid-exchanged materials were identical and observed to both be tetragonal with identical lattice constants even though the parent compounds were either tetragonal (RbSr2Nb3O10) or orthorhombic (KSr2Nb3O10). 93Nb NMR studies however revealed that the two acid-exchanged compounds had different environments for the niobium sites with the interior niobium position demonstrating drastic differences in strain. Both the symmetry and magnitude of the EFG at these positions were significantly different. In both cases, the local structure of the interior niobium site was more similar to environment observed in the parent compounds than to each other. Both niobium sites in the acid-exchanged forms show a reduction in the EFG demonstrating that the effect of the proton on bonding does translate throughout the layer. Nevertheless, the changes are not sufficient to erase the influence of the parent compound. Our studies of the nanosheets derived from these acid forms will thus need to examine the materials from numerous parent compounds to understand the subtle differences that could alter the surface acidity.
In the summer of 2008, Professor Sarah Pilkenton from
Framingham State College was supported to conduct research associated with the
synthesis and analysis of the surface acidity of the nanosheet materials that
can be produced from the layered niobates. Her research resulted in an improved exfoliation method for
the production of nanosheets. The
progress was promising and we plan to continue the collaboration to further
study the surface of the materials.
Her summer work has also helped to jump start a research project at her
institution on exfoliation methods that will include undergraduate students.
[1] X. Wang, L. J. Smith, J. Mol. Catal. A, 281, 214-218 (2008). [2] J. A. Kurzman, M. J. Geselbracht, Mater. Res. Soc.
Symp. Proc. 988, 0988-QQ08-06
(2007).