

46549-G4
Structure-Function Studies of Indoleamine 2,3-Dioxygenase (IDO)
The main objective of this project is to determine the mechanism of tryptophan (Trp) oxidation catalyzed by the heme-enzyme, Indoleamine 2,3-Dioxygenase (IDO). We proposed to do this by preparing reactive mixtures of IDO, Trp and O2, and then characterizing any transient heme iron-oxo/Trp intermediates using resonance Raman (RR) spectroscopy. The main approach we proposed and have been pursuing for generating the transient intermediates is rapid mixing. A significant portion of our time has therefore been spent on the development and testing of the continuous flow rapid mixer.
We have been developing a microfluidic mixing chip in collaboration with Dr Guodong Sui in our department, who is a microfluidics expert and has agreed to collaborate with us. The chips consist of a block of PDMS polymer mounted to a thin glass substrate (Fig. 1A and 1B). Microchannels are imprinted into the polymer for the fluid to flow. The fluid entry ports are connected to a multi-syringe pump, which drives the reactant solutions into the mixer. Several chip designs have been tested, including “Y” (Fig. 1A), “T” and cross-shaped (Fig. 1B) mixers. With the cross-shaped design the enzyme solution is pumped through the central channel whilst the Trp/O2 solution flows via two channels on either side of it. The flow is laminar and mixing is achieved by diffusion between the parallel channels. This is illustrated in Fig. 1C where a red and a blue dye are combined in the mixing chip. To modify the speed of mixing we are currently working on chips that create chaotic flow at the point of entry of the fluids.
One problem we
needed to address is that the PDMS has a strong
Raman signal that can interfere with the enzyme measurements. We are able to
avoid this problem by focusing the Raman excitation laser in the center of the
fluid channel using a confocal microscope. The confocality of the microscope
allows us to reject signals coming from above and below the focal plane that can
Our design and
testing of the microfluidic rapid mixer for RR spectroscopy
is almost complete, with optimal
sample flow
rate and concentration parameters currently being determined.
This phase of the project took longer than expected, mainly due to problems for
several
months this year with the UV exposure system used for microfluidic chip
manufacture. This problem has now been resolved and we anticipate that
measurements on reactive mixtures of IDO/Trp/O2 will commence soon.
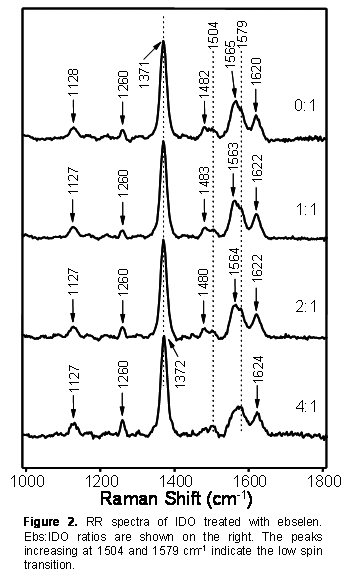
This year we also
performed experiments that examined the interaction of hydrogen peroxide and
ebselen with IDO. Ebselen is a redox-active, anti-inflammatory drug. Hydrogen
peroxide and ebselen are potentially important compounds for the regulation of
IDO activity in vivo through oxidative reactions. We used RR spectroscopy to
examine the effect of these compounds on the heme active site of IDO. In both
cases the heme iron was converted from high spin to low spin (Fig. 2). We also
performed circular dichroism spectroscopy and kinetic studies of IDO treated
with ebselen. We found that ebselen acts as a non-competitive inhibitor of IDO
activity and that it induces protein secondary structural changes by reacting
with cysteine residues on the protein.
Our research on IDO is revealing not only how this important
enzyme performs its catalytic reactions but also how
it may be regulated in vivo. The rapid mixing experiments combine cutting edge
microfluidic technology with the powerful spectroscopic technique of RR
spectroscopy. There are only a few examples of this type of experiment currently
in the literature. It is a novel approach that can reveal for the first time
the transient intermediates involved in the oxidative reactions catalyzed by IDO.
The technique can be applied to other enzymes as well. This research grant has
enabled the P.I. to develop valuable new methodologies and technical
capabilities that can be applied for years to come in the lab. The project has
also provided valuable financial support and research experience for a talented,
young Ph.D. student, Tito Sempertegui.
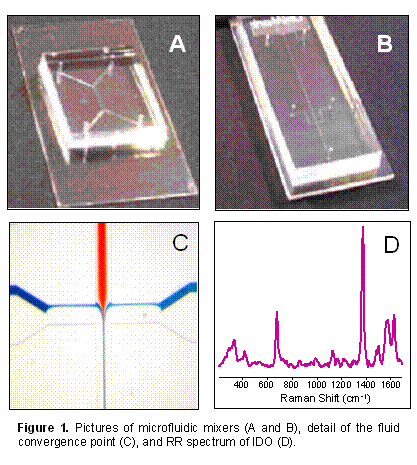 arise from the surrounding PDMS.
This works only with the right combination of
microscope objective magnification, confocal hole-size and microfluidic channel
depth. We tested channels with depths between 25 and 200
mm,
confocal hole sizes
from 100 to 1000
mm
and various microscope objectives. We
found that a 100
mm
channel depth with a 100x (NA 1.3) microscope
objective and 200
mm
confocal hole size is the best combination. We have successfully measured the
RR
arise from the surrounding PDMS.
This works only with the right combination of
microscope objective magnification, confocal hole-size and microfluidic channel
depth. We tested channels with depths between 25 and 200
mm,
confocal hole sizes
from 100 to 1000
mm
and various microscope objectives. We
found that a 100
mm
channel depth with a 100x (NA 1.3) microscope
objective and 200
mm
confocal hole size is the best combination. We have successfully measured the
RR
 spectrum of IDO flowing
through the chip using 413.1 nm laser excitation (Fig. 1D).
spectrum of IDO flowing
through the chip using 413.1 nm laser excitation (Fig. 1D).