

40958-GB7
Studying Structural Parameters Important to the Topochemical Polymerization of Butadiene
The objective
of this project is to design and study the topochemical 1-4 polymerization of
butadienes within crystals and gain insight into structural influences on the
polymerization process. The outcome of these reactions depend more on the
molecular packing than on the inherent reactivity of the molecules making up
the crystal. As a result, topochemical reactions have not gained wide spread
use as a synthetic tool. To be successful, butadiene monomers must be
organized into stacks with the proper orientation and spacing between reactive
atoms for polymerization to occur. In order to increase the scope and general utility
of topochemical and solid state reactions it is necessary to have general supramolecular
tools that can be transferrable to the design of a wide range of substrates. Our
previous work has demonstrated that we can successfully employ a supramolecular
strategy that has been used in the design of topochemical diacetylene polymerizations
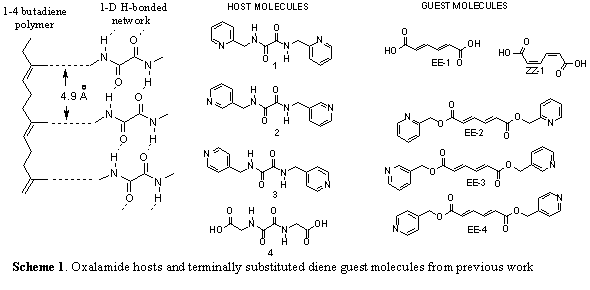
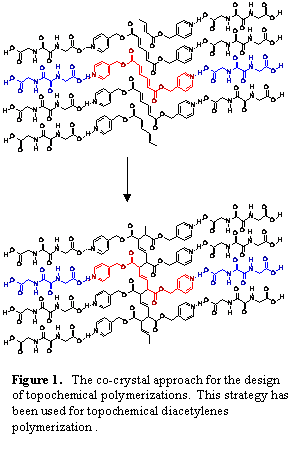
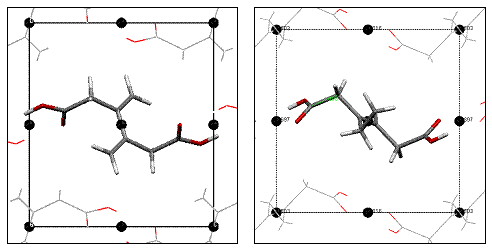
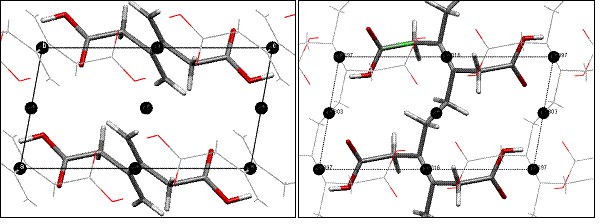
The co-crystal strategy employed involves crystallizing host and guest molecules where host molecules (1-4) organize diene guest molecules (EE-1-4) for solid state reactivity (scheme 1). The oxalamide host molecules form predictable and persistent 1-D H-bonded networks. These host molecules were chosen since the H-bonded networks they form have a characteristic lattice spacing of 4.8-5.1 Å. This spacing matches the molecular repeat of the desired 1-4 polymer. The co-crystals that result will contain guest molecules organized into stacks with a lattice repeat equivalent to that of the host network (4.8-5.1 Å). This spacing of monomers insures that the polymer product can fit within the host lattice. We believe that this strategy will minimize the lattice strain associated with the polymerization process (fig.1).
 |
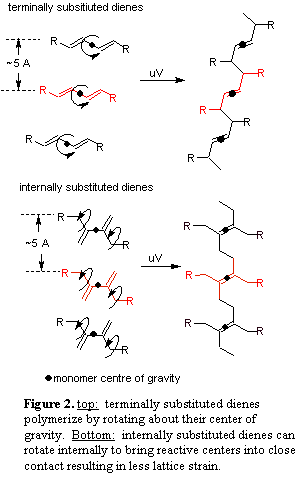
Current work is focused on 2,3-disubsituted butadiene guests molecules. There is currently nothing in the literature on the solid-state reactivity of internally substituted dienes. We believe these systems will make excellent candidates for the topochemical polymerization. Topochemical polymerizations require that reactions occur with the least amount of atomic motion.

We have synthesized 3,4-bis(methylene)hexanedioic
acid 1
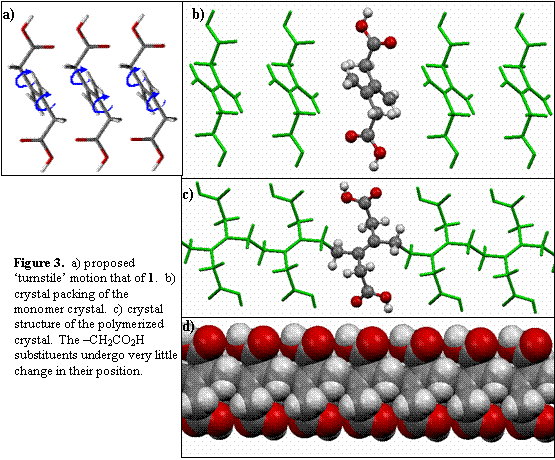
We are currently correlating changes in IR spectra and PXRD in an attempt to be able to correlate changes in molecular structure (IR data) with the movement of miller planes in the PXRD. It is hoped that this will allow us to track the small atomic movements involved in topochemical processes. We think this will be especially useful for solid state reactions where a crystal structure for the product is not obtainable.
P21/c a = 4.7483 Ǻ b = 9.729 Ǻ β = 100.45 c = 8.9186 Ǻ V = 405.172 Ǻ3 P21/c a = 4.803 Ǻ b = 8.897 Ǻ β = 100.3 c = 9.016 Ǻ V = 379.066 Ǻ3
 | |||
 | |||
|
J. Am. Chem. Soc.; 1995; 117(48); 12003-12004. c) Lauher, J. W.; Fowler, F. W.; Goroff, N. S.
Acc. Chem. Res.; (Article); 2008; 41(9); 1215-1229.