45592-AC5
Investigation of Catalysis by Nanoparticles and Metal-Ion Complexes Embedded in Multilayer Polyelectrolyte Films
Development of
effective
heterogeneous catalysts is vital for creating environmentally benign
reactions in
which expensive catalytic materials can be conveniently recycled.
This
research aims at synthesizing selective, heterogeneous catalysts
through
immobilization of metal nanoparticles in multilayer polyelectrolyte
films both on
alumina particles and in membranes. Nanoparticles are attractive
catalysts
because of their high surface area and tunable electronic properties,
and
embedding these materials in polyelectrolyte films prevents particle
aggregation and restricts access to catalytic sites to impart
remarkably high
selectivities.
During the
past year, we demonstrated that the catalytic selectivity of Pd
nanoparticles varies dramatically with particle size. These
catalysts are
prepared using layer by layer deposition of a poly(acrylic acid)-Pd2+/polyethyleneimine
film on alumina particles. Subsequent reduction of Pd2+
to Pd(0) by
NaBH4 yields nanoparticles embedded in the polyelectrolyte
film. Surprisingly,
turnover frequencies (TOFs) for the hydrogenation of monosubstituted
unsaturated
alcohols increase with decreasing average nanoparticle size, whereas
multisubstituted unsaturated alcohols show the opposite trend.
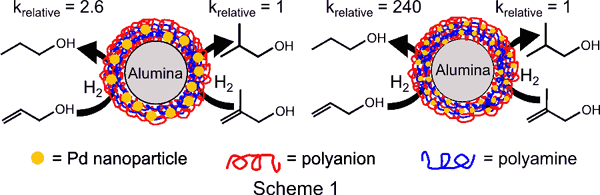
Hence, as shown
in scheme 1, the ratio of TOFs (moles hydrogenated/mole Pd/time) for
the hydrogenations
of allyl alcohol and 2-methyl-2-propen-1-ol is 240 with average Pd
nanoparticle
diameters of 2.2 nm and only 2.6 with average particle diameters of 3.4
nm. These
remarkable selectivities with the 2.2-nm particles are an order of
magnitude
higher than the selectivity of Wilkinson's catalyst, the prototypical
homogeneous catalyst for such reactions. Although, a number of
studies showed
that the activity of nanoparticles is a strong function of particle
size,
studies of selectivity are particularly attractive for investigating
nanoparticle
reactivities because comparison of relative reaction rates avoids the
question
of whether increased activity is simply related to an increased surface
area to
volume ratio.
We also investigated
the use of Pd
nanoparticles as catalysts for the hydrogenation of nitroaromatic
compounds to the
corresponding anilines. Such reactions are important because
functionalized aniline
derivatives are synthetic intermediates in the production of dyes,
herbicides,
pesticides, and pharmaceuticals. Unfortunately, in complex
syntheses, reduction
of other functional groups in nitroaromatic compounds can also lead to
undesired
side products. As an initial example, we studied the
hydrogenation of
nitrobenzaldehyde because this reaction can potentially yield a number
of
different products as shown in Scheme 2. Table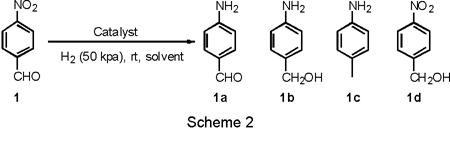
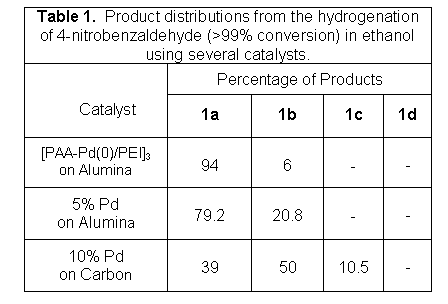 1 shows the distribution of products
from the hydrogenation of nitrobenzaldehyde with both commercial
catalysts and Pd
nanoparticles in 3-bilayer poly(acrylic acid)/polyethyleneimine films
[PAA-Pd(0)/PEI]3. Notably, in ethanol,
the desired compound, 1a, is less than 50% of the product when
using a
Pd on C catalyst. The yield of 1a is somewhat higher with
commercial 5%
Pd on alumina, but only the [PAA-Pd(0)/PEI]3 catalyst gives
>90% 1a
in the product. The [PAA-Pd(0)/PEI]3 catalyst
also shows nearly complete
selectivity for the hydrogenation of nitro groups in the compounds
shown in
Scheme 3. Future work aims at assessing the effects of
nanoparticle size on a much
wider range of catalytic reactions.
1 shows the distribution of products
from the hydrogenation of nitrobenzaldehyde with both commercial
catalysts and Pd
nanoparticles in 3-bilayer poly(acrylic acid)/polyethyleneimine films
[PAA-Pd(0)/PEI]3. Notably, in ethanol,
the desired compound, 1a, is less than 50% of the product when
using a
Pd on C catalyst. The yield of 1a is somewhat higher with
commercial 5%
Pd on alumina, but only the [PAA-Pd(0)/PEI]3 catalyst gives
>90% 1a
in the product. The [PAA-Pd(0)/PEI]3 catalyst
also shows nearly complete
selectivity for the hydrogenation of nitro groups in the compounds
shown in
Scheme 3. Future work aims at assessing the effects of
nanoparticle size on a much
wider range of catalytic reactions.
The funding from this
grant was vital in starting this work and supporting two
graduate students, Somnath Bhattacharjee and David Dotzauer. The
area of
catalysis is a relatively new one in the Bruening group and support
from PRF
has helped us gain needed expertise to begin working in this
area. PRF funding
has served as seed money for current proposals.