

46049-GB7
Design and Synthesis of Chiral Nematic Liquid Crystal Twist Agents
Background
Liquid crystals are anisotropic fluids that possess varying degrees of long-range order normally associated with crystals, while retaining the fluidity of true liquids. The most successfully commercialized LC material for displays is the chiral nematic (N*) phase wherein rod-shaped molecules adopt a supramolecular helical ordering with the helix axis orthogonal to the molecular long axis. N* materials are typically formed by doping chiral compounds ("twist agents") into achiral nematic phases as shown in Figure 1.
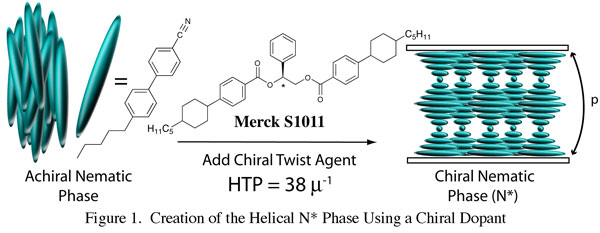
The figure of merit for a chiral dopant's ability to induce helicity is helical twisting power (HTP) defined as 1/p(c)(r) where p is helical pitch in microns, c is concentration of dopant, and r is the enantiomeric excess. We have initiated a synthetic program to help understand the relationship between dopant conformation and HTP. Thus, we synthesized several N* twist agents structurally analogous to the commercially available Merck S1011 utilizing L-amino acids as the chirality source. These diamide targets were chosen partly because of the conformationally restricted nature of the amide bond. Ultimately we hope to qualitatively predict HTP by examining low energy conformer populations in silico.
Synthesis of New Dopants
The versatile starting material in our syntheses is the well-known nematogen 5CB (compound 1b) and the phenyl-cyclohexyl analog 1a. Basic hydrolysis gave the carboxylic acid 3 or amide 2, with the latter yielding amine 5 via a Hofmann rearrangement. Using methanol as solvent in the Hofmann afforded the methyl carbamate 4 which in turn facilitated clean mono-methylation of amine 5 over two steps. Standard peptide coupling conditions with HBTU gave the L-proline and L-alanine derived dopants with both pentyl-biphenyl and phenyl-trans-pentylcyclohexyl mesogenic cores (compounds 8a, 8b, 11a, and 11b). Unfortunately, these compounds proved to be sparingly soluble in the nematic host E7. Thus, we explored N-methylation as a means to disrupt intermolecular hydrogen-bonding and enhance solubility. Direct methylation of 8 and 11 with sodium hydride/methyl iodide gave the N-methylated twist agents 9 and 12 in good yield. We also developed a more modular approach by methylating carbamate 4 followed by basic hydrolysis to give mono-methyl amine 15a in good yield; importantly, this two step approach avoids the unwanted N,N-dimethyl byproduct produced when directly methylating amine 5. The decreased nucleophilicity of the N-methyl amine 15a rendered standard HBTU-mediated peptide coupling ineffective. Subsequently, we employed the highly reactive N-Fmoc acid chloride of alanine to achieve coupling with the N-methylamine 15a in acceptable yields. The use of sodium hydride with preformed amides, as well as the use of thionyl chloride with Fmoc amino acids gave us concern about possible racemization. We were quite satisfied to find that two unique samples of 12a, one from each route, gave identical values of HTP in E7. Thus, we are confident that neither route induces racemization.
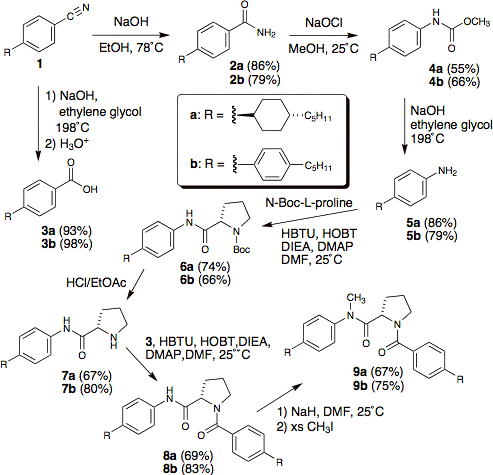
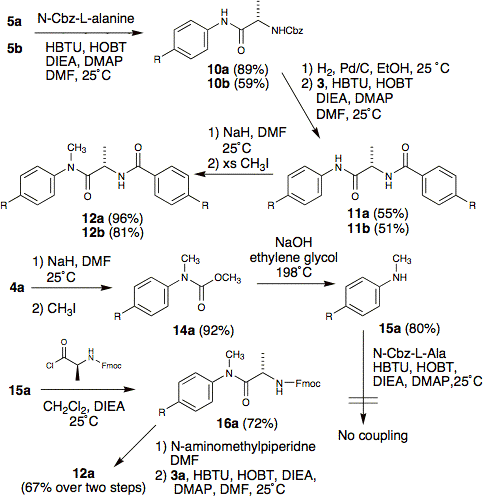
Helical Twisting Power of Diamide Dopants
The HTP of the soluble N-methylated dopants were significant, ranging from 24.6 Ą-1 (12a) to 13.5 Ą-1 (12b) for the two alanine-based dopants. The proline-based dopants had HTP values of 16.3 Ą-1 (9a) and 20.8 Ą-1 (9b). These data do not yet point to a clear correlation between the amino acid or the mesogenic core and HTP. We are currently pursuing the synthesis of the N,N'-dimethylated alanine derivatives as well as incorporation of an alkoxybiphenyl mesogenic core.
Conformational Analysis of N-Methyl Amides and Unexpected Mesogenicity
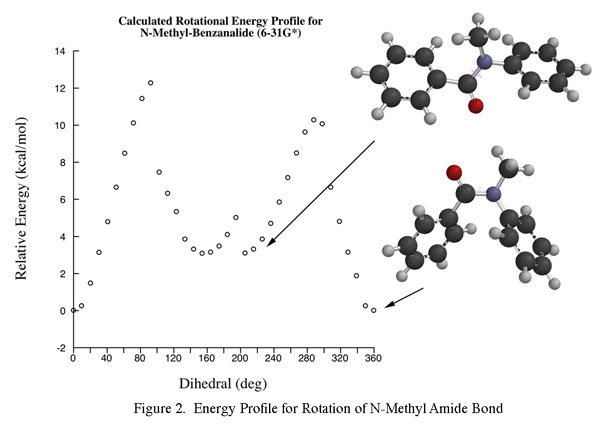
While N-methylation increases the solubility and lowers the melting points of these diamide dopants, calculations indicate that N-methylation also has a significant influence on low energy conformer populations. Significantly, the benzene ring attached to the amide nitrogen is strerically prohibited from coplanarity with the amide carbonyl. For example, this leads to a calculated minimum energy conformer (6-31G*) for N-methylbenzanilide that is bent, leaving the methyl group syn-coplanar with the amide carbonyl as shown in Figure 2. However, rotation about the amide bond reveals a relative minimum about 3.5 kcal/mol higher in energy with a roughly linear geometry.
We further investigated the effect of N-methylation by comparing the melting points of amides RL1 and RL2 shown in Figure 3. Not surprisingly, RL2 is a high-melting Smectic A material. Given that RL1 would presumably favor an overall bent conformation, we were surprised to discover that RL1 possesses an enantiotropic mesophase between 140 and 154¹ C.
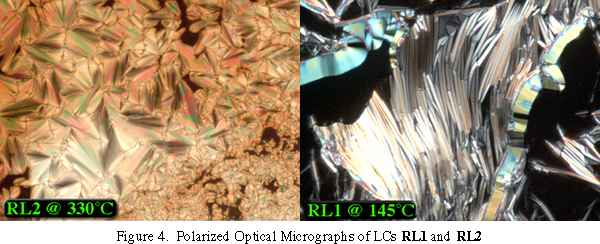
Polarizing optical micrographs of RL1 and RL2 are shown in Figure 4. While RL2 displays a focal conic texture typical for a Smectic A, the texture for RL1 remains mysterious. X-ray diffraction experiments indicate a layered phase with a layer spacing of approximately 30 ü. These results suggest that N-alkyl amides may provide a route to novel bent-core liquid crystals and several new derivatives are being synthesized.
Undergraduate Participation
Five undergraduates worked on this project. Two are now employed in the chemical industry, one is pursuing a teaching career, and one is in the Ph.D. program at UCLA.