47267-AC10
Fundamental Studies of Nano-Scale Catalysts In Direct Internal Reforming Solid Oxide Fuel Cells
The purpose of this project is to develop a fundamental understanding of the catalytic and electrochemical properties of a class of solid oxide fuel cell anodes that exhibit nanometer-scale metal precipitation on internal surfaces during fuel cell operation. To that end, we have investigated lanthanum strontium chromium oxide (LSCr) compositions doped with various precious or transmission metal dopants. These dopants have been selected not only on their hydrocarbon reforming and electrochemical properties, but also on their price and price stability, so that the anodes developed as part of this work can be employed in commercial solid oxide fuel cell applications. As shown in Figure 1, these price considerations have eliminated rhodium as a viable LSCr dopant, despite our initial proposal made when rhodium was much cheaper, and focused our attention on LSCr doped with ruthenium, palladium, and cobalt.
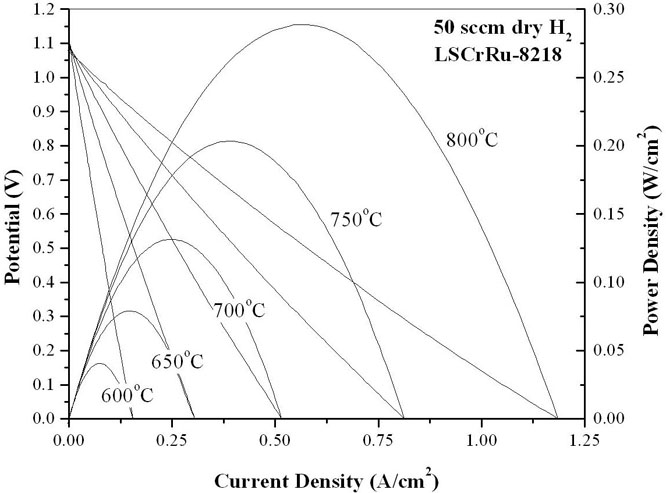
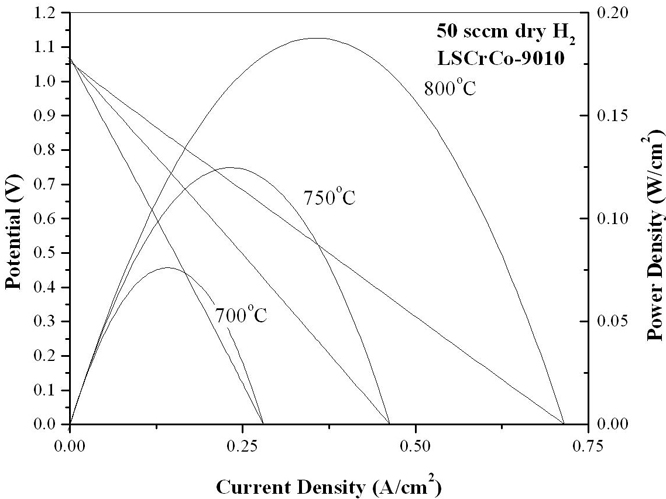
Samples of La0.8Sr0.2Cr0.82Ru0.18O3-x (LSCr82-Ru18), La0.8Sr0.2Cr0.90Co0.10O3-x(LSCr90-Co10), La0.8Sr0.2Cr0.82Co0.18O3-x(LSCr82-Co18), and La0.8Sr0.2Cr0.70Co0.30O3-x(LSCr70-Co30), have been synthesized and confirmed to be phase pure via x-ray diffraction. La0.8Sr0.2Cr0.80Pd0.20O3-x, has also been synthesized, but was not phase pure. As shown in Figure 2, transmission electron microscopy on these samples has shown that at the 18 mol% dopant concentration level, ruthenium nano-clusters form when exposed to reducing conditions (800°C for 1 hr in 50 sccm of dry H2) while cobalt nano-clusters do not. Preliminary transmission electron microscopy, gas chromatography, and fuel cell performance data also suggest that this reducing treatment is insufficient to produce metallic cobalt nano-clusters, even when LSCr is doped with up to 30% cobalt. Figure 3 shows the performance of two LSCF-CGO|LSGM|Doped-LSCr fuel cells after stabilization at 800°C in dry hydrogen. The cell with the LSCrRu anode displayed a 57% performance improvement during the first 24 hours of operation due to Ru nano-particle nucleation, whereas the LSCrCo composition showed little change with time and yielded a 50% lower post-stabilization power output than the LSCrRu anode cell. The evaluation of higher cobalt levels, longer reduction times, and alterative dopants is ongoing. Further, a high temperature conductivity relaxation setup that will allow for a direct measurement of the surface exchange coefficient for these materials is nearly ready for use.
Figure 1- Average Monthly Metal Prices
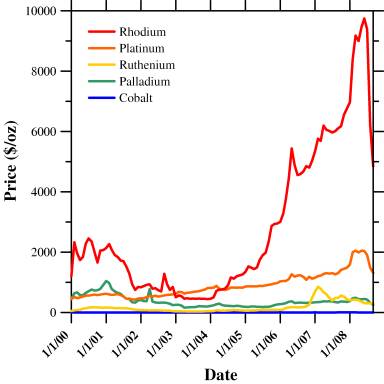
Figure 2- Transmission Electron Micrographs of Reduced LSCr82-Ru18 (left) and LSCr82-Co18 (right)
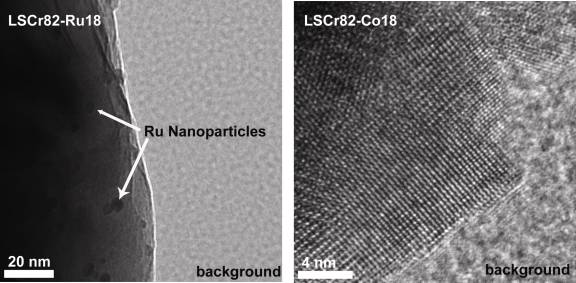
Figure 3-Solid Oxide Fuel Cell Performance Data