44308-GB3
Synthesis and Photochemical Characterization of Hexacoordinate Silicon(diimine)3 Complexes
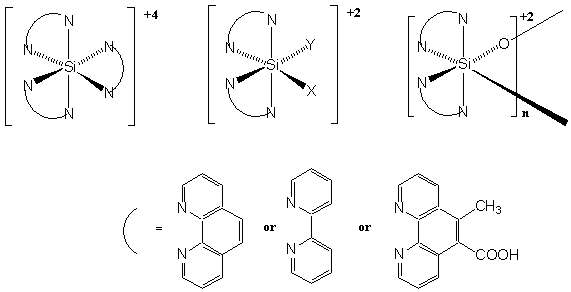
The goal of this project is to synthesize a library of hexacoordinate silicon complexes with multi-dentate polypyridyl ligands and to study their electronic and optical properties (Figure 1). During the first year we focused on the synthesis and purification of several homoleptic complexes, including Si(bipyridine)3(PF6)4, Si(phenanthroline)3(PF6)4, Si(terpyridine)2(PF6)4, and some heteroleptic complexes including Si(phen)2(OMe)2(PF6)4, Si(bipy)2(OMe)2(PF6)4. All the complexes were synthesized by melting SiI4 in excess ligand following the procedures of Kummer and coworkers (Z. Anorg. Allg. Chem. 1979, 459, 145-156). The iodide salts are generally soluble in water and the +4 cation is easily precipated out of aqueous solution upon the addition of NH4PF6.
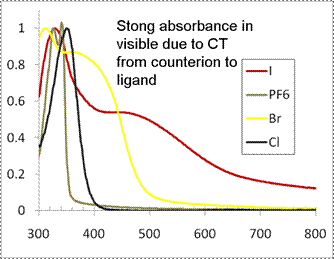
Recently, we improved the synthesis by developing a microwave technique that uses significantly less ligand, dramatically shorter reaction times, and produces a higher yield. In addition we have been studying the interesting electronic and optical properties of these complexes. While the ubiquitous Ru(diimine)32+ complexes (e.g. Ru(bipy)32+) have been the subject of thousands of papers, due primarily to the well studied photochemical, photophysical, and electrochemical properties, the chemistry and properties of the corresponding silicon analogs such as Si(bipy)30 and Si(bipy)34+ have been the subject of very few studies. In the case of the Si(bipy)34+ cation, cyclic voltammetry experiments of the Si(bipy)3(PF6)4 salt in CH3CN solution indicate 3 reversible reduction peaks and a fourth peak that results from depositing of the Si(bipy)3 neutral compound on the electrode. CV of the Si(bipy)3Cl4 salt in water also appears to have three reversible reduction peaks. The electrochemistry of Si(phen)34+ and Si(terpy)24+ cations are providing similar results, although with Si(terpy)24+ the first four reduction peaks are reversible. We have also begun to measure the spectroscopic properties and the fluorescence and fluorescence lifetimes of the compounds. Most of the complexes exhibit fluorescence at 77K and both the fluorescence and absorbance properties are very dependent on the counter-ion. UV-vis absorbance spectra of thin films of Si(bipy)3X4 (X = I, Br, Cl, PF6) (Figure 2) for example indicate the presence of a strong CT band from the counterion to a ligand based orbital, which is predicted by DFT calculations.
The structural variety and stability of hexacoordinate polypyridylsilicon compounds coupled with their strong cross-sections throughout the visible and their rich electrochemistry suggests that these complexes may have significant utility for photochemical processes.
Figure 1.
Some representative hexacoordinate silicon complexes being studied
Figure 2.
Solid-state UV-vis absorbance spectra of various Si(bipy)3+4
salts.