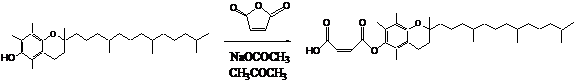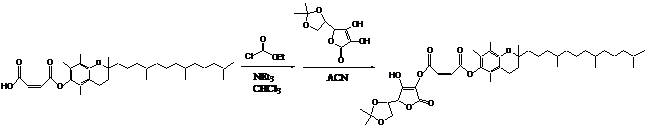Back to Table of Contents
44507-GB7
Design of Biodegradable Surfactants to Control and Manipulate Physical Properties of Polymeric Nanoparticles Made by Emulsion Evaporation Method
Cristina Sabliov, Louisiana State University
Main
accomplishments
The
ultimate goal of the present research is to design bio-friendly surfactants
(BFS), derivatives of alpha-tocopherol, to be used in the synthesis of
biocompatible and biodegradable nanoparticles of controlled physical properties
(size, morphology).
More specifically, the following
objectives were proposed:
- To synthesize bio-friendly surfactants by covalently linking ascorbic acid to alpha-tocopherol derivatives
- To synthesize PLGA nanoparticles using the emulsion-evaporation method in the presence of the new, bio-friendly surfactants.
- To characterize the PLGA nanoparticles in terms of size, size distribution, morphology, and toxicity.
Of the
three objectives, objective 1 was met during 2006-2007, as anticipated. A
bio-friendly surfactant was synthesized from alpha-tocopherol, ascorbic acid,
and maleic anhydride and the solubility, surface tension in water, and
antioxidant activity of the surfactant were determined. The synthesis of the
biofriendly surfactant (BFS) was continued in 2007-2008, with the goal of
finding the appropriate conditions under which this new compound could aid in
formation of nanoparticles (Objectives 2 and 3 of the grant).
Bio-friendly
surfactant (BFS) synthesis (Objective 1)
The
BFS was made following the synthetic pathway shown below. The final structure
was confirmed by C-NMR.
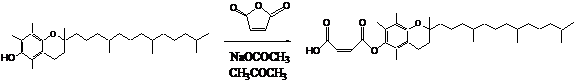
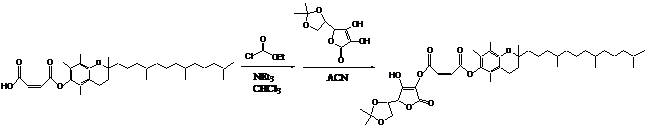

Nanoparticle
synthesis (Objectives 2 and 3)The low
water solubility of BFS prohibited its use as a surfactant in the emulsion
evaporation method, as originally proposed. In an attempt to modify emulsion
evaporation (by replacing water with the water-ethanol mixture) such that BFS
could still be used as a surfactant under the same methodology, it was found
that BFS did not form micelles in ethanol, and its solubility in this solvent exceeded
300 mM. BFS's enhanced solubility in ethanol triggered an interest in elucidating
its behavior in another organic solvent- acetone, which also proved to be a
good solvent for our component. Based on these findings, it was decided that an
alternative method could be applied to make nanoparticles with aid from BFS,
namely nanoprecipitation. In this method, acetone was used as the organic
solvent. BFS and the polymer poly(lactic-co-glycolic) acid (PLGA) were
dissolved in acetone, and the organic phase was dispersed, under sonication in
water. Rapid diffusion of acetone to the continuous water phase resulted in
precipitation of the PLGA/BFS nanoparticles. The nanoparticles exhibited a
spherical morphology right after synthesis; the resuspended nanoparticles
following freeze-drying lost their spherical shape as visualized by TEM, but
still maintained their size (Figure 1).
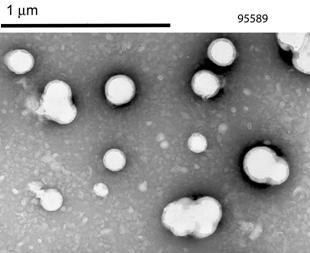
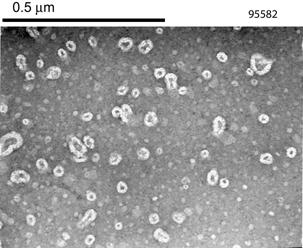
Figure 1. Morphology of the nanoparticles prior (left) and after
freeze-drying (right) as visualized by TEM
The freshly
synthesized particles averaged in size 165 nm as measured by DLS, with a
polydispersity index of 0.15 (Figure 2).
Zeta potential was very negative and measured -65mV at a pH of 6.6 (Figure 2). BFS oriented itself on the
surface of the particles with the ascorbic acid moieties toward the water, and
the alpha-tocopherol lipophilic tail toward the center of the particle. The
presence of PLGA aided in formation of such structures and contributed to the
negative charge of the particles.
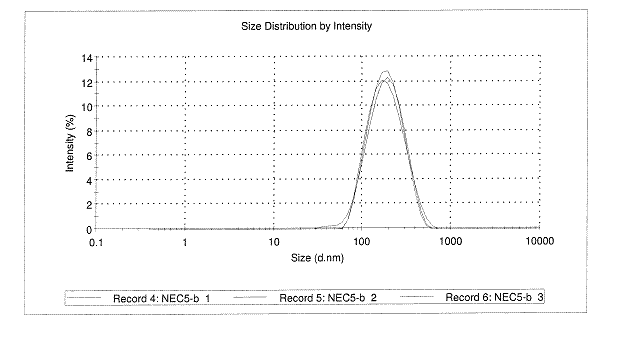
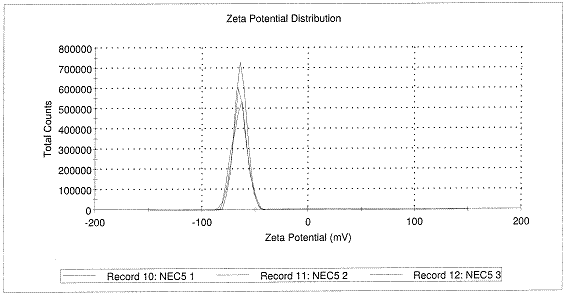
Figure 2. Three measurements of size (top) and
zeta potential (bottom) of nanoparticles made with 11% w/w BFS by
nanoprecipitation method
Future plans
It is our
goal to improve the size and polydispersity of the formed nanoparticles by the
selected method-- nanoprecipitation. We also plan to explore other methods to
make particles where the use for a biofriendly surfactant with niche properties
such as those of alpha-tocopherol-ascorbic acid is required. Span80 is a
surfactant of similar properties with BFS that is commonly used to form
particles based on w/o emulsions. It is expected that, due to its similar
structure, BFS could as well be applicable in similar methods, possibilities
which will be explored during the next 12 months. A one year no-cost extension
was requested and approved by ACS to allow for the time required to meet our
newly set objectives.
Impact of PRF-G funding on the PI's
professional development
Funding from
ACS PRF-G was instrumental in the development of the PI's program. Supplies, stipends
for undergraduate and graduate students were made possible from this award.
While a proposal on synthesis of novel biofriendly surfactants for use in the
preparation of polymeric nanoparticles was not successfully funded by the NSF
Chemistry division, novel ideas were generated and preliminary data was
acquired during the duration of the PRF-G proposal which will be vitally needed
for future grant submissions. A grant proposal on Vitamin E delivery was funded
by USDA in 2008 and part of its success can be attributed to ACS support. Also,
most importantly, a cohesive research team was created as a result of working
on this grant (Dr. Dolliver-chemist, Dr. Norwood-physicist, Dr.
Nesterov-chemist, and Dr. Sabliov-biological engineer) and the strong relationships
developed during the last two years will surely help the PI's future
professional development, as well as contribute to other team members'
success.
Back to top