

43683-G7
Effects of Particle Additions to Hydrogels
<>Overview
Hydrogels are water-swollen interconnected polymer networks that can be covalently or physically cross-linked. Polyacrylamide-based hydrogels have been extensively studied for biomaterials applications such as cellular substrates. While their high liquid content mimics the permeability of the extracellular matrix surrounding a cell, the liquid component of hydrogels also results in low mechanical stiffness values (modulus on the order of kPa) compared to that of many biological tissues (modulus of MPa – GPa). In the present study we examined the effects of both hydrogel composition as well as particle additions on the mechanical properties of hydrogels.
Rheological Results Scaling Behavior of Hydrogels of Varying Compositions and Volume Fractions
We examined the effects of the total hydrogel volume fraction on the shear elastic modulus for varying hydrogel compositions. Three hydrogel compositions were chosen for the polyacrylic acid-based system, 25/75, 50/50 and 100/0. Four compositions of the polyacrylate-based hydrogels were chosen for volume fraction studies: 0/100, 15/85, 25/75, and 100/0. These compositions were tested across a range of polymer volume fractions (Фp) from 0.02 to 0.25 using strain amplitude sweeps ranging from 0.001% to 4500% at a fixed frequency of 6 rad/s. The shear storage modulus value for each sample was taken from the linear viscoelastic regime of the strain sweeps and is plotted in Figure 1(a). Generally, an increase in either the 1) total hydrogel volume fraction or 2) polyacrylamide percentage resulted in a corresponding increase in the shear storage modulus. The strengthening effects of polyacrylamide become more evident as the modulus values exhibit increasing divergence with polymer volume fraction. Of all the hydrogels, pure polyacrylate was the weakest exhibiting the minimum G′ values across all polymer volume fractions. To better elucidate composition and volume fraction effects on the mechanical properties, the data from Figure 1(a) is re-plotted as a log-log plot in Figure 1(b) to reveal any scaling behavior.
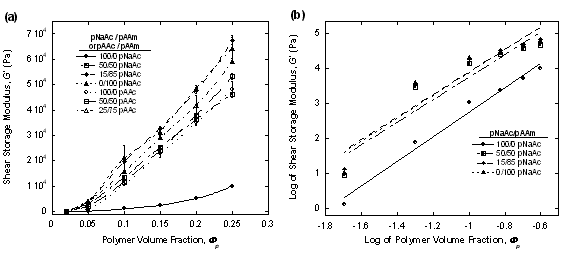
Figure 1. (a) Linear and (b) log-log plots of average shear storage modulus
values as a function of total polymer volume fraction (jp) for
polyacrylic/polyacrylamide (pAAc/pAAm) and polyacrylate/polyacrylamide
(pNaAc/pAAm) hydrogels with varying volume percentage ratios. Each modulus value was taken from strain
value of ~0.34% from strain sweeps.
Based on the fitted curves shown in Figure 1(b), slopes of 3.2 and 3.5 were determined for the polyacrylate and polyacrylic-based systems respectively. While this value deviates from deGennes' reported value of 2.25 for ideal polymer chains in the semi-dilute regime1, our results closely match those of recent work by Meyvis2. We hypothesize that the deviation from deGennes predictions stems from the effects of dangling polymer chain ends that do not contribute to the elasticity response of the network to applied stress. A manuscript of this study is currently in preparation.
Effects of Particle Additions on Hydrogel Stiffness
Next, we added colloidal particles of varying surface chemistries to test the strengthening effects of colloidal fillers. Since the hydrogel network is negatively-charged, we tested the effects of different surface chemistries (e.g. negatively-charged carboxylated polystyrene lattices and positively-charged spermine-grafted polystyrene lattices) to generate varying degrees of attractive or repulsive interactions at the hydrogel-colloid interface. We had predicted that cases involving cationic particles would allow the colloidal particles to act as macro-scopic crosslinking agents and would stiffen the hydrogels. We found in fact, that additions of 0.79 mm-diameter particles did not stiffen the matrix modulus compared to pure hydrogels without embedded particles. To increase the effective interfacial area between the hydrogel matrix and particle fillers, we then explored using smaller 0.12 mm-diameter particles; however, particle size effects were minimal. Interestingly, particle additions in several cases (e.g. carbon nanotubes) appeared to actually weaken the hydrogel by reducing its shear storage modulus. While dispersing the individual particles throughout the matrix simply does not appear to strengthen the hydrogels, we hypothesize that perhaps aggregated particles would more effectively stiffen the matrix by providing a more continuous network of interactive fillers.
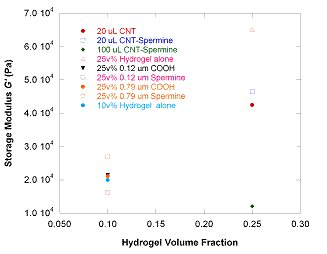
Figure 2. Shear storage modulus values of hydrogel-colloid composites at varying polymer hydrogel volume fractions. The 15/85 polyacrylamide/polyacrylate hydrogel has a net negative charge due to the acrylate copolymer. Particle surface chemistries ranged from anionic carboxylated and cationic spermine-grafted polystyrene lattices to rod-like carbon nanotubes. The frequency was fixed at 6 rad/sec.
References
1. de Gennes, P. Scaling Concepts in Polymer Physics. 1979, Ithaca: Cornell University Press.
2. Meyvis, T.K.L. et al J. Rheol. 1999. 43:933-950.