

45406-AC6
Solvent-Enabled Reactivity of Negative Ions
The outcomes of chemical reactions are subject to the multitude of interactions characteristic of condensed environments. The research project supported by this ACS PRF grant examined the role of solvent molecules in photoinduced chemical processes, making use of cluster anions to attain a molecular view of chemistry. The broad objective of this work, particularly relevant to the mission of the ACS Petroleum Research Fund, was to investigate solvent-enabled chemistry in anionic environments, spotlighting the reaction pathways that are either enhanced or made possible entirely by the presence of the solvent. Specifically, we examined two types of effects:
(1) The formation of new chemical bonds under the effect of interactions with the environment;
(2) Solvation-induced shift from the photoinduced electron detachment towards the breaking of chemical bonds.
First, using photoelectron imaging as a tool for examining
the electronic structure and bonding motifs, we studied the a variety of
cluster systems. The intermolecular
interactions in clusters often lead to the formation of new covalent bonds, in
some important cases resulting in the competition and coexistence of the
covalent dimer anion cluster cores and the monomer anion cluster cores. This was found to be particularly true for
the cluster anions of CO2, CS2, OCS, and others. We have demonstrated that the balance between
different cluster species is affected to a large degree by the homogeneous
solvation and heterogeneous hydration.
The photochemistry is also affected by the intermolecular
interactions with the environment. Absorption of light by a negative ion
generally leads to electron detachment or promotion to excited anionic states,
which serve as gateways to chemical transformations. Since the excess electron
orbitals, due to their diffuse nature, are particularly sensitive to the
surroundings, the excess electron is usually an active participant in chemical
dynamics and a sensitive probe thereof.
In particular, the branching between the electron detachment and anion
dissociation can be tuned using stepwise solvation.
As a specific example, we used photoelectron imaging to
investigate the coexistence of several isomers of (CS2)2-.
The bands in the photoelectron images and spectra were assigned to the
CS2-·CS2
ion-molecule complex and two different dimer-anion structures with covalent
bonding between the two monomers. The
isomer distribution and anionic reactivity were found to depend sensitively on
the presence of water in the precursor gas mixture, which has important
implications to chemistry in wet of humid environments. One of our important findings was the evidence
that the presence of water enhances the formation of the global-minimum dimer
structure, rather than the metastable structure corresponding to the
ion-molecule complex. However, in the
larger (CS2)n- and (CS2)2-(H2O)m clusters, the population of the covalent-dimer core
structures diminishes drastically due to more favorable solvent interactions
with the monomer-anion core.
In another PRF supported study, we investigated the
structural properties and underlying reactivity of [O(N2O)n]- cluster anions generated via electron
bombardment of a pulsed
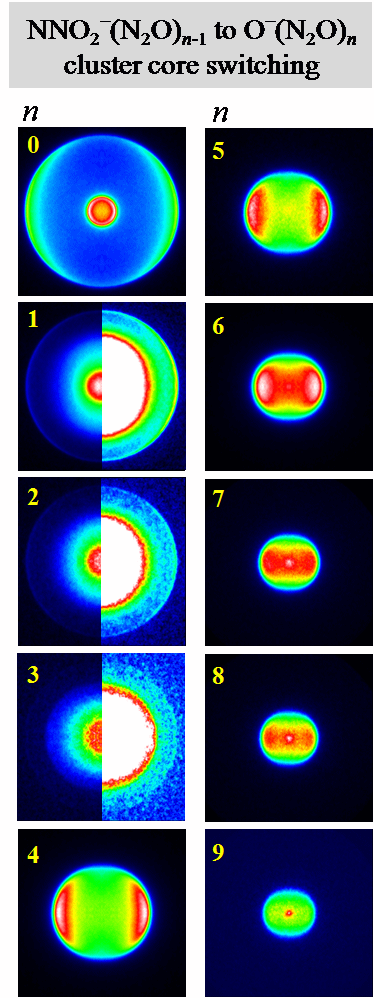
supersonic expansion of pure N2O gas. The photoelectron images obtained in this
study at 355 nm are shown in the Figure, where the laser polarization direction
is set vertical in the plane of all images.
Depending on cluster size, the images give evidence of two dominant
core-anion structures, corresponding to the NNO2-(N2O)n-1
and O-(N2O)n cluster anions. In
agreement with previous studies, the n
= 1 anion has a covalently bound (Y-shaped) NNO2- structure. The striking new result that
came out of our work is that the NNO2- core was found to persist only in the clusters with n up to 3. For n These results provide prominent demonstrations of and insight
into the role played by the solvent in controlling the structure of anionic
compounds and their corresponding photochemistry. The results and conclusions
drawn from our studies of solvent-enabled chemistry enhance our fundamental
understanding of intermolecular interactions and their roles in chemistry of
condensed environments.
The work in this project was carried out by graduate students
in the Sanov group. These students were
provided not only with support for their graduate education, but also with rich
training opportunities in the multi-faceted experimental techniques and the
process of scientific discovery. Two of
the students supported in part by this project (Luis Velarde and Terefe
Habteyes) earned their Ph.D. degrees in 2008 and moved to postdoctoral
positions in other research groups.
 To accomplish these objectives,
we employed a combination of tandem time-of-flight mass-spectroscopy and
photoelectron imaging. Experiments were
carried out on several cluster anion systems, including the pure and hydrated
CO2 of CS2 cluster anions, NNO2-(N2O)n-1 and O-(N2O)n
cluster anions, as well as others.
To accomplish these objectives,
we employed a combination of tandem time-of-flight mass-spectroscopy and
photoelectron imaging. Experiments were
carried out on several cluster anion systems, including the pure and hydrated
CO2 of CS2 cluster anions, NNO2-(N2O)n-1 and O-(N2O)n
cluster anions, as well as others.