

45854-GB10
Proton Conduction Mechanisms in Superionic Phases of Phosphate-Based Solid Acids
This study is aimed at uncovering the atomic-level structures and dynamics that enable the sharp increase in the proton conductivity of the title materials upon heating above a temperature threshold. Understanding the microscopic mechanisms of this so-called superprotonic behavior is important not only because of its potential application in the field of alternative energy (e.g. for use in fuel cells that can function at temperatures above 100°C), but also from a fundamental standpoint, as such highly efficient proton conduction mechanisms are likely to play a central role in processes that occur in a large variety of other physical, chemical, or biological systems.
To achieve the goals of this research we designed a two-step approach. First, we use temperature-resolved synchrotron X-ray diffraction to determine the evolution of the room-temperature phases upon heating and uncover the structural modifications that accompany the transition to the superprotonic state. Once the crystal structures of the superprotonic phases are known in detail (including possible disorder), we employ neutron spectroscopy to investigate the proton diffusion through these structures. In our proposal we also anticipated that an important aspect of this research program would be the active involvement of students, especially at the undergraduate level. Finally, as this is a GB (starter) grant, preliminary results are to be used to apply for additional funding to continue this research.
We have made significant progress in all the above-mentioned directions.
Scientifically, we obtained the following results. For CsH2PO4 we first determined that the lattice parameters of the room-temperature monoclinic phase vary smoothly upon heating to a temperature right below the superprotonic transition threshold. This indicates that no structural or chemical changes occur within this temperature interval. Then, using high-pressure methods, we demonstrated that a polymorphic monoclinic-cubic transition occurs upon further heating. We collected data of enough quality to carry out Rietveld refinements that revealed subtle structural details of the high temperature superprotonic phase (e.g. dynamic disorder and a slight distortion of the PO4 tetrahedra). We published these findings in the Journal of Chemical Physics in the fall of 2008. Figure 1 (below) shows the high-pressure synchrotron X-ray powder diffraction pattern together with the best Rietveld fit, as well as the structure of the high-pressure high-temperature CsH2PO4 phase.
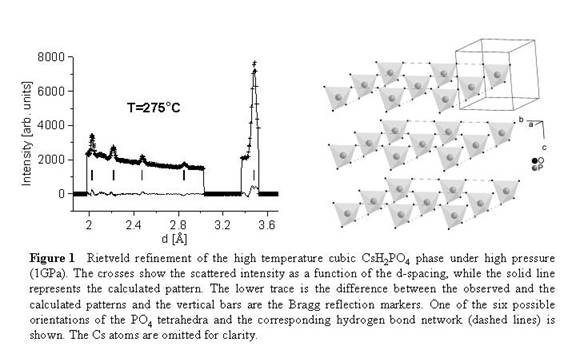 For
RbH2PO4 we carried out temperature-resolved synchrotron
X-ray diffraction under both ambient and high-pressure conditions (1 GPa). Our data clearly show the
first polymorphic (orthorhombic-to-monoclinic) transition at T~95°C and, upon further heating, also reveal
evidence of the modification of the monoclinic phase at T~320°C, which is the same temperature where the
transition to the superprotonic state had been
observed under a pressure of 1 GPa.
For KH2PO4 we found that the intermediate-temperature
monoclinic phase simply melts upon heating above 260°C under ambient pressure, or 325°C
under 1 GPa of pressure,
which explains the absence of the superprotonic
behavior for this material.
For
RbH2PO4 we carried out temperature-resolved synchrotron
X-ray diffraction under both ambient and high-pressure conditions (1 GPa). Our data clearly show the
first polymorphic (orthorhombic-to-monoclinic) transition at T~95°C and, upon further heating, also reveal
evidence of the modification of the monoclinic phase at T~320°C, which is the same temperature where the
transition to the superprotonic state had been
observed under a pressure of 1 GPa.
For KH2PO4 we found that the intermediate-temperature
monoclinic phase simply melts upon heating above 260°C under ambient pressure, or 325°C
under 1 GPa of pressure,
which explains the absence of the superprotonic
behavior for this material.
There has been active student involvement in this research, both at the M.S. and at the undergraduate level. One student successfully defended his M.S. thesis and another has completed his first year of graduate work and plans to graduate in the spring of 2009 with a thesis on high-pressure high-temperature transitions in RbH2PO4. During the summer of 2007 an undergraduate student was awarded a Supplement for Underrepresented Minority Research (SUMR) scholarship to carry out research under my supervision. Following this research experience, and his graduation with a B.S. in physics in the summer of 2008, the SUMR scholar decided to pursue graduate studies in physics and is currently a member of my group working towards a M.S. in physics on the same project on which he carried out research within the SUMR program.
In addition, I successfully used the preliminary data obtained using the ACS-PRF funding to apply for additional financial support for the continuation and development of this research program. In 2008 I received a Norman Hackerman Advanced Research Program Award from the Texas Higher Education Coordinating Board, as well as a Cottrell College Science Award from the Research Corporation. This clearly demonstrates the strong and positive impact of the ACS-PRF GB-type grant on the development of my career.
During the next year, we plan to complete the second step of our proposed research plan by carrying out neutron spectroscopy experiments aimed at investigating the microscopic aspects of the proton dynamics in the superprotonic phases of these solid acids. The interpretation of the neutron spectroscopy data for CsH2PO4 (including possible scenarios for the mechanisms of proton diffusion) will be greatly facilitated by our detailed knowledge of the crystal structure of the high-temperature phase of this material. In addition, as our research develops, we plan to continue increasing the student participation, especially at the undergraduate level.