47084-AC3
New Homogeneously Catalyzed Dehydrogenation and Hydrogenation of Polar Bonds. Esters and H2 from Alcohols, Hydrogenation of Esters, and Related Reactions
PRF report
During the first year of the project we concentrated on the preparation of new pyridine-based pincer ligands and complexes, and the study of their reactivity, aiming at extension of the concept of metal-ligand cooperativity based on aromatization-dearomatization of the pyridinic ring, and developing catalytic reactions based upon it. Our previous work with iridium and ruthenium complexes has been based on PNP and PNN-type pincer ligands, which are air sensitive due to the phosphine group. We have now developed air stable thiolate and sulfoxide ligands and complexes thereof.
(1) In looking for alternatives for phosphine ligands, which have similar coordinating characteristics without being air sensitive or toxic, we prepared pincer ligands with sulfinyl groups. They can act as S or O donors according to electronic and steric requirements and they are better σ-donors than nitriles and good p-acceptors (S coordination).
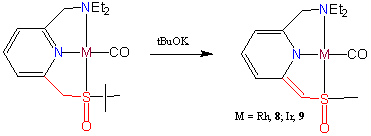
The new pyridine based sulfoxide pincer ligand 1 (2-(diethylaminometyhl)-6-(tert-butyl-sulfinylmethyl)pyridine = S(O)NN) reacts cleanly with Rh2(COE)4Cl2 to form the neutral water and air stable RhI complex Rh(S(O)NN)Cl 1. The cationic complexes [Rh(S(O)NN(NCCH3)][BF4] 2 and [Ir(S(O)NN)(COE)][BF4] 5 were obtained by the reaction of 1 with the appropriate metal precursors. The corresponding carbonyl complexes [Rh(S(O)NN)(CO)][PF6] 4 and [Ir(S(O)NN)(CO)][BF4] 7 exhibit nCO in the IR spectra which shows that ligand 1 is a relatively poor σ-donor ligand compared to the more common PNP-type ligands, resulting in rather electron poor metal centers. The carbonyl compounds can be deprotonated to the remarkably stable dearomatized complexes Rh(S(O)NN*)(CO) 8 and Ir(S(O)NN*)(CO) 9. DFT studies on 8 revealed a high electron delocalization over the pyridine ring system and the sulfur atom, which explains the high stability of 8 towards re-protonation. Complete protonation of 8 was achieved in acetic acid.
(2) Hemilabile, PNN phosphine- based pincer complexes of late metals, in
which a "hard" amine or ether pincer "arm" is capable of de-coordination,
exhibit a desirable balance between stability and reactivity, providing new opportunities
in stoichiometric and catalytic reactions. In the case of the
dialkylsulfide-based SNS pincer ligands, hemilability of both "arms" of the
bis-chelating ligand is possible. While pyridine-based SNS complexes were
reported, we are unaware of such examples of iridium. We have developed the synthesis,
structure, fluxional behavior and reactivity of new Ir(I) and Rh(I) complexes
based on a pyridine-based tBuSNS ligand. Interestingly, the Ir(I)
and Rh(I) carbonyl complexes exhibit metal-metal interactions in the crystal.
The
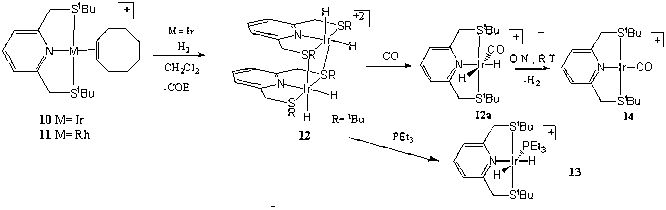
cationic, pincer-type complexes (SNS)Ir(COE)+BF4- (10) and (SNS)Rh(COE)+BF4- (11) complexes were prepared, and their
structure and reactivity were studied. They are fluxional at room temperature
as a result of "arm" hemilability, which can be frozen at low temperatures.
Reaction of complex 10 with H2 resulted in a dimeric dihydride complex (SNS)Ir(H2)+BF4- (12) in which the
sulfur atoms bridge between two metal centers. The Rh complex 11 did not react with H2. Both the carbonyl
complexes (SNS)Ir(CO)+BF4-
(14) and (SNS)Rh(CO)+BF4- (15) show differences in the IR
stretching frequencies in solution vs. solid states, which are a result of
uncommon metal-metal interactions between square planar d8 systems
in the solid state. Complexes 11, 12, 14 and 15 were
structurally characterized by X-ray crystallography. A network of hydrogen
bonds involving the BF4- counter anion and hydrogen atoms
of complex 14 was observed.