44304-GB4
Pyrrole Amides and Their Use as Building Blocks for the Preparation of Anion Receptors
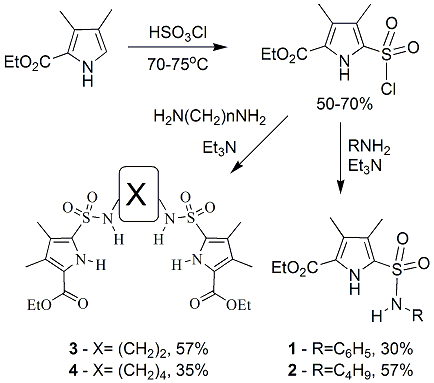
This proposal focuses on the preparation of novel pyrrole-amide molecular architectures and their use as receptors for both anions and neutral molecules. The objectives of this project are: develop synthetic methodology that allows for the “large-scale” preparation of a diverse library of pyrrole-amides and prepare dipyrrinone analogs containing the pyrrole-amide motif for use as anion receptors. Objective 1- Develop synthetic methodology that allows for the “large-scale” preparation of a diverse library of pyrrole-amides: As documented in the previous report, the primary work related to objective 1 has been met, and the results have been accepted for publication in Synthetic Communications. In addition, the objective was expanded to include the preparation of pyrrole a-sulfonamides. Specifically, the synthesis and initial molecular recognition studies of four simple pyrrole a-sulfonamide derivatives, 1-4, is reported. All target molecules were prepared from readily available 2-carboethoxy-3,4-dimethyl-1H- pyrrole, 5, see Scheme 1. Initially, chlorosulfonation of 5 in neat chlorosulfonic acid and subsequent destruction of the excess chlorosulfonic acid lead to the isolation of the pyrrole sulfonyl chloride derivative, 6, in 50-70% yield. Sulfonyl chloride 6 was reacted with both mono and diamines in dry CH2Cl2 to give the target molecules, 1-4, in reasonable yields (35-57%). The yields vary in large part due to the hydrolysis of the sulfonyl chloride moiety of 6 due to the presence of any moisture, which leads to the corresponding sulfonic acid. | ||||||||||||||||||||||||||||||
Scheme 1. Syntheses of sulfonamide target s 1-4 | ||||||||||||||||||||||||||||||
Molecular recognition studies were conducted via 1H NMR with 1-4 and various guests (chloride, bromide, hydrogen sulfate and nitrate as their respective tetra-n-butylammonium salts and benzoic acid) in dry CDCl3 at 25oC. Analysis of the titration data found that the data fit only a 1:1 host–guest binding mode in a linear regression model and revealed the stability constants in Table 1. Binding stoichiometry was confirmed via Job Plot analysis. The pyrrole NH(s) experienced a 0.5-1.2 ppm change in chemical shift upon addition of the exogenous guest while the sulfonamide NH(s) generally experienced slightly larger changes in chemical shift (1.5-2.3 ppm). Table 1. Stability Constants (M-1)of 1-4 with various guest speciesa
a - Studies conducted in CDCl3 at 25oC & all anions were used as their respective tetra-n-butylammonium salts; b - Benzoic Acid; nb - no evidence of binding | ||||||||||||||||||||||||||||||
Examination of the stability constants reveals that the analogs with only a single pyrrole and sulfonamide present (1 and 2) gave substantially smaller Ka’s than the bis-analogs (3 and 4). Additionally, hydrogen sulfate had a significantly larger binding affinity for both 3 and 4. Preliminary investigation suggests that the sulfate hydoxyl group is involved in a hydrogen binding interaction with the ester carbonyl of the pyrrole moieties. Further studies are underway to investigate the hydrogen bonding interactions involved in the formation of the complex. This work is currently being prepared for publication. | ||||||||||||||||||||||||||||||
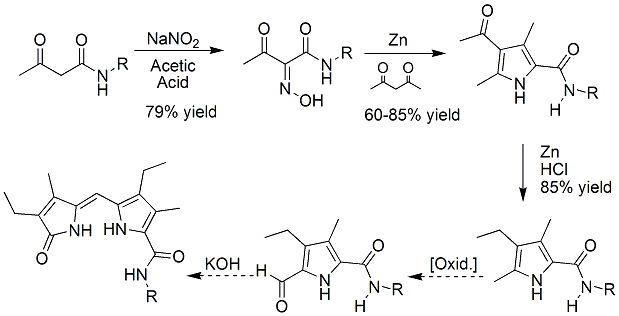
Scheme 2. Route 1 for preparation of dipyrrinone amides. | ||||||||||||||||||||||||||||||
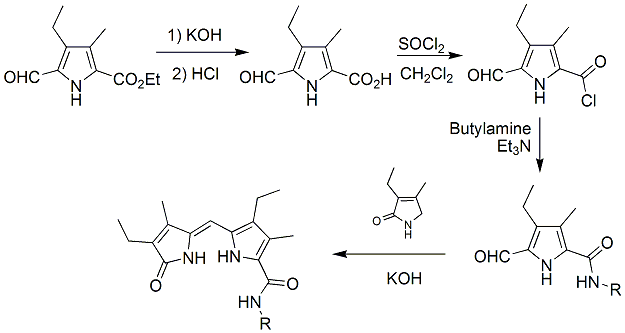
| Objective 2: Prepare dipyrrinone analogs containing the pyrrole-amide motif for use as anion receptors - Preliminary work on preparing the dipyrrinone amides has begun in our labs. We are developing a synthetic route to prepare dipyrrinone amides on large scale with control of the amide substituents (N- groups). The most direct synthetic method involves preparation of the dipyrrinone 9-carboxylic acid derivative and subsequent amide formation. Unfortunately, this method has proven unsuccessful to this point. Thus alternate routes are being explored. One potential route involves the direct preparation of the required pyrrole a-amides from acyclic starting materials, and subsequent formation of the dipyrrinone. This route is currently being explored, and is based on the work of objective 1. At this point, we are still working out the conditions needed for the selective oxidation of the a-methyl to the desired aldehyde. Although there are known conditions for such transformation in other pyrrole analogs, there are no conditions which have been successful on pyrrole a-amides. This transformation has yet to be successful and will require some additional method development work to complete the syntheses via this route. As such, we have also been pursuing an alternate route to preparing dipyrrinone amides, Scheme 3. For the alternate route, the needed pyrrole a-amide was made from the corresponding acid chloride which already contained the aldehyde functional group. Once prepared, this intermediate was condensed with the appropriate pyrrolinone under basic conditions to give the desired product. Only preliminary studies have been conducted, but this route looks promising. We have prepared only small quantities of the dipyrrinone amide using this method, but have experienced purification issues. It is anticipated that this project will be completed, and will result in an eventual publication or two. | ||||||||||||||||||||||||||||||
Scheme 3. Route 2 for preparation of dipyrrinone amides. |