44182-GB1
Functionalized Diaziridines as Potential Electrophilic Aziridinating Agents
The generation of easily manipulated synthetic intermediates facilitates complex molecule synthesis.1 The ease of formation and extensive reactivity of epoxides2 has underscored their synthetic utility.3,4,5 Aziridines possess a similar potential6 but have yet to become ubiquitous intermediates due to lack of a general aziridination method (Figure 1). Diaziridines, the nitrogen equivalent of dioxiranes, represent a promising class of aziridinating agents7 (Figure 2). However, their lack of applicable usage reflects the inherently lower reactivity of unsubstituted diaziridines when compared with dioxiranes. Thus a general application of diaziridines awaits a practical activation method.
Modeling studies by Houk and Armstrong8
have implicated the diaziridine as a promising aziridinating agent.
Specifically, N-Silyl, N-trifluoroacetyl, and N-alkyl oxaziridines and
diaziridiniums have been identified as potentially useful agents for the
aziridination of olefins. Given this background,
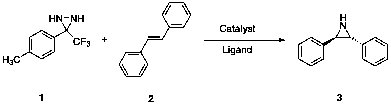
3-(4-methyl)-3-trifluoromethyldiaziridine seems to be a promising aziridinating
agent, as it is electron-poor. Various reaction conditions have been employed
to investigate proper conditions for universal aziridination method.
A variety of catalysts to promote have
been investigated. Copper catalysts were anticipated to be potentially
effective aziridination catalysts due to the affinity of copper for nitrogen9.
It was hoped that copper will bind to one of the nitrogen atoms of the
diaziridine to facilitate heteroatom transfer; however, issues may arise if the
affinity of copper is so great that the –NH group is not released.
Copper(I)salen complex or
copper(I)triflate catalysts were combined with stilbene and diaziridine in
ratios of moles of catalyst to moles of diaziridine of 1:1, 1:1.3, 1:2, and
1:10. However, each of these spectra showed a singlet peak at approximately 10
ppm which was not present in the 1H NMR spectra of stilbene,
diaziridine, the salen ligand complex, and triflic acid, a possible byproduct.
However, neither the imine, aziridine, diaziridine, stilbene, or catalyst were
detected via mass spectrometry, which indicates possible product decomposition.
Copper (II) catalysts, including
Cu(II)OTf and Cu(tfac)2, were also used in a few of the
aziridination attempts. Reactions catalyzed by there copper complexes gave
white solid products; however, as in many of the previous reactions, there were
no peaks that correpsonded to the desired aziridine. Since the 13C NMR spectrum of the aziridination
product contained no such peaks, the reactions employing both of these copper
(II) catalysts were concluded to be unsuccessful.
Thus far, the aziridination attempts
using n-BuLi has yielded inconclusive results10. The 1H
NMR spectrum of this product showed singlet peaks at 4.02, 3.80, and 3.30 ppm,
which fall in the 3-4 ppm range corresponding to protons H2 and H3
of the aziridine. The spectral data gathers indicates that that aziridination
reaction may have been successful when catalyzed by n-BuLi; however, further
analysis is needed to determine the identity of the aziridination product.
1 McCoull, W.; Davis, F. A.,
Synthesis, 2000, 1347.
2 For reviews see: (a) Murray, R. W., Chem. Rev. 1989, 89, 1187. (b) Frohn, M.; Shi, Y., Synthesis, 2000, 1979. (c) Xia, Q. H.;
Ge, H. Q.; Ye, C. P.; Liu, Z. M.; Su, K. X., Chem. Rev. 2005, 1603.
3 Tanner, D. Chiral aziridines.
Preparation and stereoselective transformations Angew. Chem. Int. Ed. Engl.
1994, 33, 599.
4 Hanson, R.; Sharpless, K. B., J.
Org. Chem. 1986, 51, 1922.
5 Johnson, R. A.; Sharpless, K.
B.,Catalytic Asymmetric Synthesis A. Ojima, Ed.; New York, 1993; pp159-202.
6 Atkinson, R. S., Tetrahedron 1999, 55, 1519.
7 Hori, K.; Sugihara, H.; Ito, Y. N.;
Katsuki, T., Tetrahedron Letters 1999, 40, 5207.
8. Washington, I.; Houk, K.; Armstrong, A. J.Org. Chem. 2003,
68, 6497-6501.
9. Voet, D.; Voet, J. Biochemistry.Wiley, 2004.
10.