
Back to Table of Contents
44691-G3
New Strategies for Incorporating Magnetic Anisotropy into Single-Molecule Magnets
Matthew P. Shores, Colorado State University
The
synthesis of new single-molecule magnet (SMM) candidate materials requires the
balancing of many factors—high nuclearity, magnetic coupling (J), large ground state spin (S) and magnetic anisotropy (D)—most of which compete with each
other. Our projects in the second year of PRF support have focused on
increasing D in exchange-coupled
clusters by preparing molecules and ligand systems with topological anisotropy.
The hypothesis is that enforcing molecular shape anisotropy will predictably drive
magnetic anisotropy. (1) Transition metal ethynylbenzene
chemistry. Our goal was to expand known
Fe(III)-tris(ethynylbenzene—TEB) complexes[1] to achieve discotic SMMs. We prepared the Fe(III)-containing di- and trinuclear
core complexes 1 and 2 by oxidation of their Fe(II) analogues (only
the neutral dinuclear complex was known) (Figure 1). The magnetic exchange interactions
displayed in 1 and 2 are as predicted: strong antiferromagnetic
coupling in 1, and ferromagnetic interactions for 2 (J = –133 and +29 cm–1 from
MAGFIT;[2]
spin Hamiltonian = –2J(Si·Sj)).
These values are promising for isolating high-spin ground states, and the
presence of replaceable chloride ions means that 1 and 2 are
poised for growth to higher nuclearity species, both necessary for the development
of SMMs.
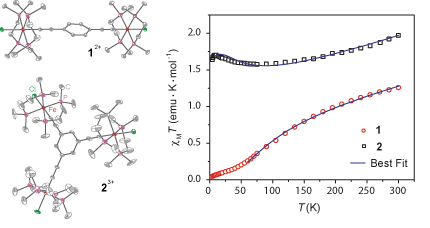
Figure 1. Structures of [(dmpe)4Fe2(TEB)Cl2]2+
and [(dmpe)6Fe3(TEB)Cl3]3+ at 40 %
ellipsoids (no H); DC magnetic susceptibility data for 1 and 2 (H = 1000 Oe).
(2) Uranium(IV) ethynylbenzene complexes.
Exploiting known triamidoamine
phenylacetylide complexes,[3] we began exploring uranium
magnetochemistry. The purpose of our initial study was to check for exchange
coupling between U(IV) species with trigonal bipyramidal coordination. If
exchange was operative, then we would assess how the bridging geometry affected
U-U interactions. We prepared the di- and trinuclear U(IV) ethynylbenzene
complexes [(NN'3)2U2(m-DEB)], [(NN'3)2U2(p-DEB)],
and [(NN'3)3U3(TEB)] (3-5); they
and the mono-phenylacetylide 6 were characterized by FT-IR, UV-Vis, and X-ray
analyses (example of 3 in Figure 2). Magnetic susceptibility data appear
to be consistent with a U(IV) non-magnetic ground state,[4] which we hoped to avoid with
trigonal bipyramidal complex geometries. However, use of a subtraction scheme[5] suggests the
presence of U-U magnetic interactions. Fits to the corrected data show weak
ferromagnetic coupling for all three complexes; this is unexpected based on
analogous transition metal complexes. We are performing electronic structure
calculations in collaboration with A. Rappé (CSU) to better understand these
results. This work has produced a new actinide-based system where we can expect
to derive meaningful magnetostructural correlations.
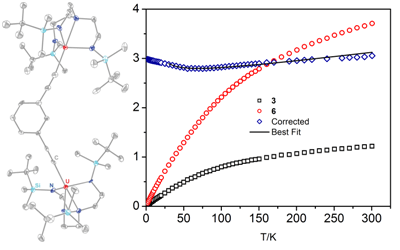
Figure 2. Crystal
structure of 3 with 40% ellipsoids (no
H); DC magnetic susceptibility data for 3
and [(NN'3)U(CCPh)] (6) (H
= 1000 Oe).
(3) Progress toward linear
metal-cyanide clusters. The ultimate in
topological anisotropy is represented by linear species. Our goal was the
preparation of trinuclear complexes to test if covalent linkage of Schiff base
complexes could improve magnetic anisotropy. We prepared the ether-linked
bis(salpn) ligand 7, and metallated with manganese(III) acetate to form
the Mn2 complex 8, which was characterized by TOF-MS, 1H
NMR and FT-IR. Reactions of 8 with hexacyanometalates and trans-M(CN)2 complexes are underway. We anticipate that the
CN-bridged trinuclear complexes linked covalently at the ends—via rigid (not
shown) or flexible (7) spacers—will allow the SMM candidates to maintain
structural integrity when redissolved, something which has not been observed
without the covalent linker.
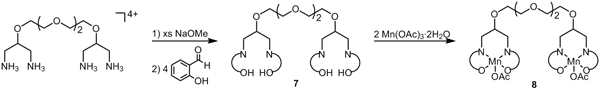
Scheme 1. Synthesis of a bis-(salpn)Mn(III) complex bound to
a sterically flexible spacer.
Summary. We have synthesized 10
dia- and paramagnetic ethynylbenzene-bridged metallodendrimer building blocks,
including several Co(III), Fe(II), Mn(III) and Cr(II) species (not discussed
here due to space limitations) that are poised for further reactivity. The
Fe(III)-containing species display predicted magnetic exchange along with
sizable J couplings, encouraging our
efforts to make higher nuclearity species. Our initial forays into the
organometallic uranium magnetochemistry have produced new multinuclear
complexes that show tantalizing evidence of U-U (ferro)magnetic interactions. Also,
a series of ligands and transition metal Schiff base complexes are poised for
assembly into linear metal-cyanide clusters. One paper has been submitted to JACS; three manuscripts are in
preparation.
([1]) Weyland, T.; Costuas, K.; Mari, A.; Halt,
J.-F.; Lapinte, C. Organometallics 1998, 17, 5569.([2]) Schmitt,
E. A. Ph.D. Thesis, University of Illinois at
Urbana-Champaign, 1995.
([3]) (a) Boaretto, R.; Roussel, P.; Alcock, N.
W.; Kingsley, A. J.; Munslow, I. J.; Sanders, C. J.; Scott, P. J. Organomet.
Chem. 1999, 591, 174. (b) Le Borgne, T.; Riviere, E.; Marrot,
J.; Thuery, P.; Girerd, J. J.; Ephritikhine, M. Chem. Eur. J. 2002,
8, 774.
([4]) (a) Almond, P. M.; Deakin, L.; Porter, M.
J.; Mar, A.; Albrecht-Schmitt, T. E. Chem. Mater. 2000, 12,
3208-3213. (b) Schelter, E. J.; Morris, D. E.; Scott, B. L.; Thompson, J. D.;
Kiplinger, J. L. " Inorg. Chem. 2007, 46, 5528-5536.
([5]) Edelstein, N. M.; Lander, G. H. The
Chemistry of the Actinide and Transactinide Elements. Springer: 2006; Vol.
4, Ch.
20.
Back to top




