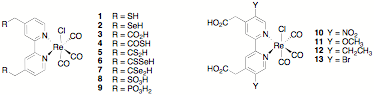45966-B6
Dependence of Rates of Interfacial Electron Transfer on Anchoring Group Structure and Dye MLCT State Energy
Because there has been no systematic study of the effects on ET rate of the identity/properties of the anchoring group or of the electronic coupling between the adsorbate and the semiconductor (through matching of the energy levels of the dye to that of the semiconductor conduction band), the proposed research entails the systematic design, synthesis, and photoinduced interfacial ET studies of a series of chlorotricarbonylrhenium bipyridine complexes substituted on the bipyridine ligand with a variety of anchoring groups (compounds 1 – 9) or substituents that modulate the energy levels of the ligand p-molecular orbitals (molecules 10 – 13).
After submission
of this proposal, we prepared, and in collaboration with Professors Tianquan
Lian and Keiji Morokuma at Emory University, we have published studies with 1, 3,
and 9: (1) to characterize contact by measuring electron transfer
rate and their dependence on anchoring group and electrode, and (2) to model these contacts by modern computation
chemistry. Our findings reveal that:
In
addition, we have also completed the synthesis of Re complex 8 and of the bipyridine ligands in compounds 4 and 5.
We have also prepared a number of intermediates in the synthesis of the ligands
in 2, 6, 7,
and 10–13. In related
studies, we and the Lian group have also published the study of the exciton
dissociation dynamics of CdSe quantum dots adsorbed with Re(CO)3Cl(dcbpy)
(dcbpy = 4,4'-dicarboxy-2,2'-bipyridine), in which excitons in CdSe dissociate
by electron transfer to the rhenium complex. The rate of this electron transfer
was determined by the size of the quantum dots, and dissociation half-time of
approximately 2.3 ps was achieved, suggesting the possibility of separating
multiple electron-hole pairs.