

45401-AC3
Titanium-Mediated and -Catalyzed Amine Allylation: Applications, Mechanism, and Implications for '[3,3]-Sigmatropic' Rearrangements Involving Metal-Ligand Multiple Bonds
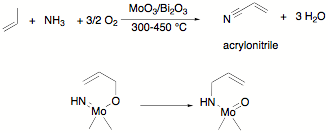
Allyl transfer to a metal ligand multiple bond substituent is a key step in many catalyses. This reaction is generally assumed to occur through [3,3]-sigmatropic rearrangement similar to a Cope or Claisen rearrangment. For example, the SOHIO process[1] for propylene ammoxidation is believed to involve the transfer of an allyl group to a molybdenum imido on the way to acrylonitrile production (Scheme 1). One proposal for the key C–N bond formation is sigmatropic migration of an allyl in this important industrial process. Alkoxide [3,3]-sigmatropic rearrangements are also drawn in the mechanism for allyl alcohol isomerization reactions catalyzed by transition metal oxides like OV(OPri)3. These reactions are used, for example, in isomerization of terpenes like linolool in the fragrance industry.[2]
Scheme 1. Propylene
ammoxidation (top) and the proposed [3,3]-sigmatropic rearrangement in the
heterogeneous catalyst that forms the new C–N bond (bottom).
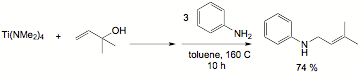
In our research, we have developed a new methodology for the
synthesis of secondary allyl amines based on a titanium-mediated process.[3]
In these reactions, an alcohol is added to commercially available Ti(NMe2)4
followed by amine. The reaction is believed to involve in situ generation of a
titanium imido alkoxide. The allyl group transfers from oxygen to nitrogen
selectively generating the secondary amine with allylic transposition (Scheme
2). In this chemistry, the rearrangement was inhibited by placing a methyl
group in the 2-position of the allyl alcohol, which should not effect a
[3,3]-sigmatropic rearrangement (Scheme 1, bottom). An alternative mechanism is
a [2 + 2]/retro-[2 + 2] cycloaddition pathway involving two fused four-membered
metallacycles. Even in the Cope rearrangement, the [2 + 2]-cycloaddition
product (bicyclo[2.2.0]hexane) is only about 18 kcal/mol in energy above the
1,5-hexadiene starting material; however, in organic systems the kinetic
barrier to reach this intermediate is extremely high due to orbital symmetries.
In organic systems, the result is that diradical or concerted [3,3]-sigmatropic
transition states are favored, which are an estimated 53 and 41 kcal/mol over 1,5-hexadiene
starting material respectively. With transition metal involvement, the symmetry
restrictions are removed and [2 + 2]-cycloaddition could become the favored
pathway, which we hopped to test in systems like the one shown in Scheme 2.
Scheme 2. Example of
the secondary allylic amine synthesis mediated by Ti(NMe2)4.
Kinetics were carried out on the 2-protio and 2-deuterio
versions of the reaction in Scheme 2. If the 2-carbon is interacting with the
titanium during transfer, one would expect an inverse secondary kinetic isotope
effect, which is exactly what was observed to a first approximation.
Unfortunately, we were unable to get definitive results due to relatively large
errors in our measurements, which may be attributed to the complexity of the
system under study. A system where a definitive complex was undergoing
rearrangement would likely give better results. Consequently, we have
redesigned the experiment with the characterizable imido alkoxides shown in
Scheme 3, which are still under study. The synthesis for the
1,2-dimethylallylalkoxide is shown in Scheme 3. The sequence begins with a
titanium imido complex available using a route developed by Lorber and
coworkers.[4]
Scheme 3. Synthesis of titanium imido allyl alkoxides.
In a parallel thrust, we have been examining the effect of stereochemistry
within the alcohol on the product distribution in reactions like the one shown
in Scheme 2. For example, when carrying out the reaction of (rac)-cyclohex-2-enol with aniline mediated by Ti(NMe2)4
we found that a single product was observed. We are planning the resolve the
alcohol to examine the enantioselectivity of the reaction. In addition, we
examined the regiochemistry of the olefin's effect on the product distribution
by starting with pure trans-1-methylcinnamyl
alcohol. Unfortunately, the reaction provided 4 products apparently due to
alcohol replacement, cis-trans isomerization, and migration of the double bond.
These studies were carried out by two undergraduates working in our laboratory.
Scheme 4. Titanium mediated additions to alcohols.
Further examination of these types of reactions may provide
additional insight into the utility of this type of titanium-mediated
transformation and its mechanism.
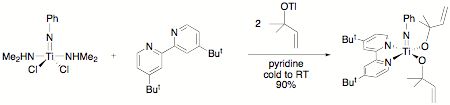
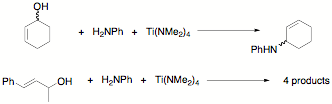
[2]. Parshall,
G. W.; Ittel, S. D. Homogeneous Catalysis,
2nd Ed.; Wiley Interscience: New York, 1992.