43041-B4
Synthesis, Metal Ion Complexation, and Emission Studies of Naphthalimide Based Fluoroionophores
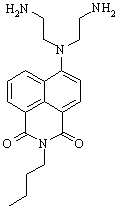
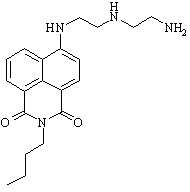
During this reporting period, we continued studies toward the synthesis of secondary amino substituted butyl naphthalimide (Figure 1). The reaction of secondary amines with halonaphthalimides has proven difficult, presumably due to the increased steric hindrance of the naphthalene moiety as compared to similar benzene systems. Several solvent systems have been studied, including ionic liquids, in an effort to increase solubility of the reagents and increase the reaction temperature. To date, we have NMR and LCMS evidence of 2°-DETA and are working to increase reaction yield in order to completely purify and characterize the product. In the next year, we plan to complete photochemical and photophysical studies of 2°-DETA in the presence of trace metal ions and compare the results with those obtained for 1°-DETA (Figure 2).
Figure 1. 2°-DETA Figure 2. 1°-DETA
Completion
of our studies of 1°-DETA and the observation that
addition of chain length and nitrogens to the ligand system does not increase
metal ion selectivity or fluorescence enhancement prompted us to examine the
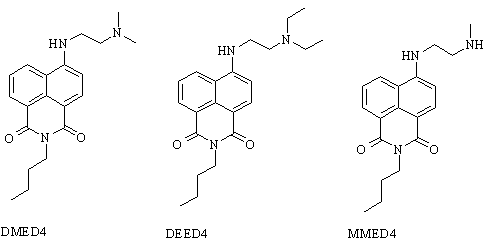
effect of alkyl substitution on the distal amine (Figure 3). 4-N',N'-Dimethylethylenediamine (DMED4),
4-N',N'-diethylethylenediamine (DEED4), and 4-N'-methylethylenediamine (MMED4)
substituted butyl naphthalimides were synthesized. Results of fluorescence enhancement studies of DMED4 and DEED4
were similar to results obtained with 4-butanediamine (BD4) substituted butyl
naphthalimide. Greater fluorescence
enhancement than for the ethylenediamine (ED4) or propylenediamine (PD4)
derivatives was recorded, but less shifting of the long wavelength emission to
the blue is observed. Selectivity for
larger metal ions such as Pb2+ is also observed for DMED4 and DEED4
as compared to smaller metal ions such as Cu2+ for ED4 and PD4. In this respect DMED4 and DEED 4 also behave
similarly to BD4.
Figure 3. 4-Alkyl
substituted butyl naphthalimides
We are continuing our studies of
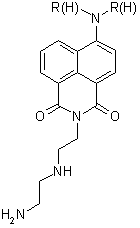
the synthesis of naphthalimides with the polyamine in the imide position
(figure 4). Coupling products of two
naphthalimides through the polyamine linker have been the major product of our
initial trials. Boc-protected
diethylenetriamine has lead to the desired major product by LCMS, but isolation
after deprotection has been problematic.
Boc-protected ethylenediamine has also resulted in good yields prior to
deprotection, but isolation difficulties of the unprotected product. Diethyldiethylenetriamine derivatized
naphthalimide is our target for the next year.
This derivative will yield the desired product without requiring
deprotection. Comparison of the results
of fluorescence enhancement studies will be compared with 1°-DETA and DEED4.
Figure 4. Diethylenetriamine imide product.
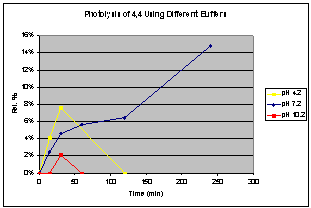
Photodegradation studies of 44
(Figure 5) are progressing. We have
conducted the reactions in buffered water at pH's of 4, 7, and 10, under N2
bubbling, irradiating with a Rayonet reactor equipped with lamps centered at
420 nm to excite into the charge-transfer band of 44. Plotting the results of different irradiation times monitored by
GCMS clearly shows a product growing in with time whose mass matches 4-amino
butylnaphthalimide (Figure 6). The
product grows in fastest at low pH and no reaction is observed at high pH. The reaction stops at low pH after
approximately an hour. Irradiation of
solution buffered to pH 7 shows product growing in for at least 4 hours, our
current time limit. Irradiation of air
saturated solution results in reactant degradation with no photoproducts
observed. Presumably the solutions are
photooxidizing. The pH dependence of the photoreaction will be examined in more
detail, as this is an important key to the mechanism of this reaction.
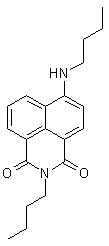
Figure 5.
4-Butylaminobutylnaphthalimide (44)
Figure 6. Photolysis results of 44
During
this reporting period, four students were directly supported by grant funds and
three students were indirectly supported through materials purchased by grant
funds. These students experienced
research activities and worked with equipment most undergraduate students do
not have access to allowing them to greatly advance in their studies toward
becoming independent chemists, which will greatly aid them in their future
endeavors. The undergraduate students
working with me this reporting period are all graduate school bound, so
research is a necessary activity to prepare them for their next phase of
training. The research project funded
during this reporting period has allowed me to continue my personal study in
chemistry and photochemistry. Research
activities are the primary means for academic chemists to remain current in the
field. Excitement for chemistry is
definitely noticed by students at all levels, not just during research.