

45416-AC7
Cure and Properties of Thermosetting Materials at the Nanoscale
Nanoscale constraint is known to have a significant impact on the thermal properties of materials. Such changes are particularly important for the use of thermosetting materials in nanoelectronic applications, as well as for the use of nanoparticle-thermoset composites. The objective of the proposed work is to investigate these effects under the well defined nanoscale constraints imposed by controlled pore glass (CPG).
During the course of this grant, we investigated the isothermal curing under nanoscale constraint of a thermosetting resin, bisphenol M dicyanate ester (BMDC), which trimerizes to form a polycyanurate network material. Differential scanning calorimeter was used to monitor the evolution of the glass transition temperature (designated Tf' since measurements were performed on heating) and the conversion during cure as a function of the diameter of the pores of a controlled pore glass matrix which is used for confinement. Both silanized and unsilanized pores were studied. In addition, sol extraction studies were performed to determine the gelation point and the sol fraction as a function of conversion for resins cured under nanoconfinement.
A Tg depression is observed for both the bisphenol M dicyanate ester monomer and the polycyanurate networks; the depression is only a few degrees for the monomer. On the other hand, the Tg depression is approximately 60 K at the smallest pore size of 11.5 nm, seemingly independent of pore surface chemistry. In addition, although the bulk materials only show one Tg, in the unsilanized pores and in the smallest silanized pores, two transitions are observed, the primary transition (Tg1) and a secondary transition at higher temperatures (Tg2). The difference between the primary and secondary Tgs increases slightly as pore size decreases, ranging from approximately 20 to 30 K for the fully-cured polcyanurate.
The structure of
the polycyanurate network for the material cured in the nanopores is suggested
to be unchanged from the bulk.
This conclusion is arrived at based on three observations: i) the Tg (or Tf')
versus conversion relationship for the material cured in the nanopores is
identical to that of the bulk; ii) the gelation point for the material cured in
the nanopores is identical to that of the bulk; and iii) the sol fraction
versus conversion relationship is unchanged from the bulk.
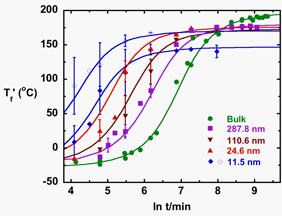 The
cure kinetics of bisphenol M dicyanate ester confined in the controlled pore
glasses has also been investigated by following the evolution of the glass
transition temperature during cure.
The results are shown in the figure as a function nanopore size for
silanized pores. As the pore size
decreases from infinity (i.e., the bulk state) to 11.5 nm, cure is accelerated. A more pronounced acceleration of the
reaction is observed in the native (unsilanized pores). It has been suggested
by Vyazovkin that the enhanced reactivity observed at nanoscale in this system may
be attributed to the increased collision efficiency of the molecules in the
vicinity of the surface due to decreased mobility. The accelerated cure under nanoconfinement is also
corroborated by the fact that the DSC onset temperature for the reaction of
initially uncured monomer confined in the CPGs decreases as pore size
decreases. Similar to the bulk
reaction studied earlier by Simon and Gillham, the reaction rate for the
confined material is well described by a second-order plus second-order
autocatalytic mechanism, but now the rate constants are a function of pore
size.
The
cure kinetics of bisphenol M dicyanate ester confined in the controlled pore
glasses has also been investigated by following the evolution of the glass
transition temperature during cure.
The results are shown in the figure as a function nanopore size for
silanized pores. As the pore size
decreases from infinity (i.e., the bulk state) to 11.5 nm, cure is accelerated. A more pronounced acceleration of the
reaction is observed in the native (unsilanized pores). It has been suggested
by Vyazovkin that the enhanced reactivity observed at nanoscale in this system may
be attributed to the increased collision efficiency of the molecules in the
vicinity of the surface due to decreased mobility. The accelerated cure under nanoconfinement is also
corroborated by the fact that the DSC onset temperature for the reaction of
initially uncured monomer confined in the CPGs decreases as pore size
decreases. Similar to the bulk
reaction studied earlier by Simon and Gillham, the reaction rate for the
confined material is well described by a second-order plus second-order
autocatalytic mechanism, but now the rate constants are a function of pore
size.