43878-G7
A Novel Methodology for the Synthesis of Main-Chain Chiral Polymers
ACS Petroleum Research Fund Annual Progress Report
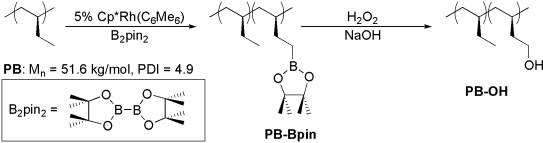
A selective and mild chemical modification of polymer can yield a new class of functional materials in a convenient manner. Especially, selective introduction of a polar group into nonpolar polyolefins, even in low concentration, could generate interesting materials that possess enhanced surface properties and improved compatibility. Utilizing recent prolific development in transition metal-catalyzed functionalization of C–H bond, the PI developed the regiospecific functionalization of the C–H bond of a commercial high-molecular-weight crystalline polyolefin, isotactic poly(1-butene) (PB; Scheme 1), via a rhodium-catalyzed alkane C–H activation/borylation with bis(pinacolato)diboron (B2pin2).
Hydroxy-functionalized isotactic poly(1-butene) (PB-OH of Scheme 1) was synthesized using transition metal-catalyzed regioselective C–H borylation at the side chain of the commercial polyolefin and subsequent oxidation of the boronic ester functionality. Functionalization up to 19 mol % of the termini of the ethyl side chain occurred without significant side reactions that could alter the polymer chain length.
Scheme 1. Regioselective functionalization of isotactic poly(1-butene).
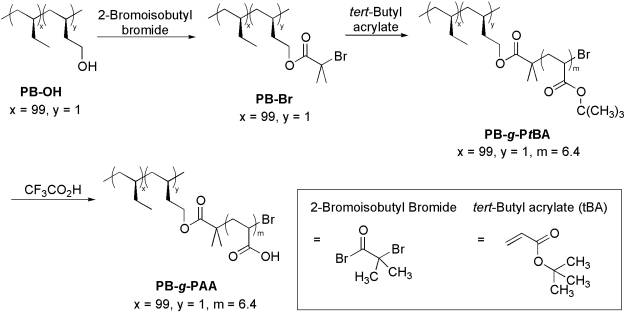
Esterification of the hydroxy group in the polymer with 2-bromoisobutyl
bromide generated a side chain-functionalized polyolefin macroinitiator. Atom
transfer radical polymerization of tert-butyl acrylate from the macroinitiator
produced a high molecular weight graft copolymer of the polyolefin, isotactic
poly(1-butene)-graft-poly(tert-butyl acrylate) (PB-g-PtBA).
Finally, the hydrolysis of the tert-butoxy ester group of PB-g-PtBA
created an amphiphilic polyolefin, isotactic poly(1-butene)-graft-poly(acrylic acid), which contained a short
carboxylic acid-functionalized polymer block at the side chain. Scheme
2. Preparation of
macroinitiator and graft copolymers of isotactic poly(1-butene).
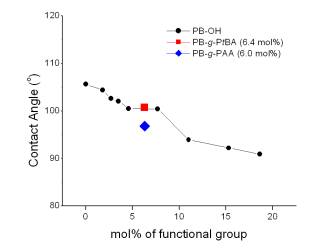
One of drawbacks of polyolefins is low surface energy, which can be indirectly studied by
measuring water contact angles of the polymers. Contact angle measurement of water on the
surface of hydroxylated PB on glass plate is plotted in Figure 1. The
unfunctionalized PB has a contact angle of 105.6o. As shown in Figure 1, the water
contact angles of PB-OHs were lower than that of PB, and the more incorporation of hydroxy
group into the polymer side chain resulted in a systematic decrease of contact angle in a
series of PB-OH. This result indicates that the functionalization led to an increase of hydrophilicity
of the polymer. Compared with the contact angle
of PB (105.6o) the 6 mol % acrylic acid-grafted PB-g-PAA exhibited a much smaller
contact angle (96.8o), which indicates the creation of more hydrophilic surface in the polymer. Figure 1. Water contact angles of a series of hydroxy-functionalized isotactic
poly(1-butene)s and graft copolymers.