45789-AC6
Infrared Spectroscopy of Cluster Anions Containing Aromatic Molecules
These first 20 months of the project have been very successful. After some work (optimizing the ion experiment for several target systems) had already been done before the start of the grant period, we were able to successfully perform a large portion of the proposed experiments. In a “sideline” experiment, we were also able to make the first steps into a new spectroscopic access to energy flow in hydrocarbon molecules.
In oxidative stress,
cells are exposed to high levels of oxygen in the form of O2 or
superoxide O2-. This is relevant in the contexts of
immune response, aging, and a variety of diseases. Consequently,
there is considerable interest in the nature of the interactions of O2
and O2- with biomolecules and models of biologically
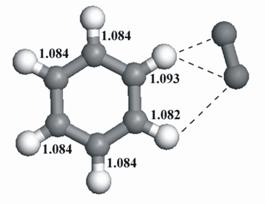
important functional groups. As a model system for aromatic groups, we chose O2-×benzene complexes. Experimentally, several
transitions due to CH stretch fundamentals and various combination bands are
observed in the 2700-3100 cm-1 region. After completion of the
experiments, we found out that the group of M. A. Johnson at Figure 1: Structure of O2-×benzene. As proposed, we also investigated the
structures of anionic hydrated fluorobenzenes. While the bonding of water to atomic and small molecular anions
has been studied for many ions and in great detail, the hydration of larger
charge distributions has not been studied at the same level. One class of
interesting model systems contains an aromatic molecule with a small positive
or even negative electron affinity, which is stabilized by the presence of a
solvent molecule, such as water. This is interesting in the contexts of anion hydration,
and of fluorination chemistry of aromatic molecules. The absorption
bands show that only one isomer of each monohydrate complex is populated in our
experiment, despite a multitude of low-lying isomers that we found in
calculations. We can assign the observed bands to an isomer where water forms a
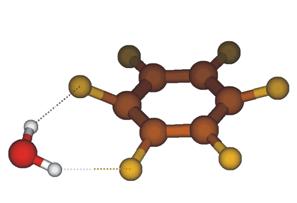
weak double ionic hydrogen bond with two neighboring CF groups (see Figure 2). The
spectroscopic motif of the binary complexes remains at lower fluorination
levels. For dihydrated hexafluorobenzene
anions, we observe hydrogen bonding between the water molecules laying the
foundation for the formation of water networks. The results were published in
2007 in J. Chem. Phys.
Figure 2: Structure of C6F6-×H2O. The most intriguing experiment was
the structural investigation of fluorinated benzenes in complexes with chloride.
This topic is interesting in the context of anion molecular recognition, as the
supramolecular chemistry of aromatic scaffolds can be tailored by substitution.
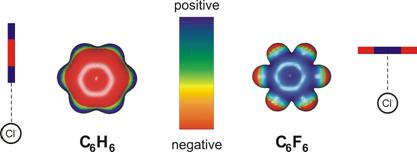
In chloride-benzene complexes, the anion binds to the benzene in bifurcated
H-bonds, while it binds to the ring in chloride-hexafluorobenzene
complexes, due to the opposite electrostatic makeup of the aromatic molecule
(see Figure 3).
Figure 3: Electrostatic potentials around
C6H6 and C6F6, and interaction with
Cl- ions. The question we addressed was how
many F substituents are needed to change the binding
motif from interaction with the CH groups in the periphery of the fluorobenzene to the ring, investigating Cl-×fluorobenzene complexes for all
fluorination levels up to pentafluorobenzene and all stereoisomers. Interestingly, the complexes remain in the
H-bonding configuration for all systems studied, implying that only chloride-hexafluorobenzene will exhibit a ring-bound structure. The
reason for this behavior lies in the increasing acidity of the fluorobenzenes with increasing fluorination, resulting in
additional stabilization of the H-bonded structure compared to a ring-bound
isomer. The results were published in J. Am. Chem. Soc. in 2007. Funds from
this grant were mainly used for the support of a graduate student (Holger Schneider). This has been the first grant during my
time as an Assistant Professor, and has been extremely valuable. It has enabled
a considerable amount of my research program. The student supported by this
grant has been working enthusiastically in this project, and his participation
in conferences has been secured by it. The results have led our group deeper into
the field of anion molecular recognition, and we hope to attract more outside
funding in this area.