42704-B5
Experimental Study of Transport Phenomena and Film Instabilities of Evaporating Multicomponent Fuel Films
Background
This project concerns the evaporation of liquid films composed of multiple hydrocarbon components (to simulate gasoline) and is motivated by the debate regarding the significance of fuel films to hydrocarbon emissions from direct-injection, spark-ignition (DISI) engines. The goals of this research are 1) to improve the understanding of the roles of the thermal and mass transport processes of film evaporation, 2) to provide a thorough characterization of the changing film composition during evaporation, and 3) to obtain experimental data taken under well-specified conditions representative of internal combustion engines that may be used for model validation. While the motivation of the program is the practical problem caused by fuel films in DISI engines, we are interested generally in the fundamental principles that govern film evaporation and therefore will study film evaporation under a wide variety of conditions, not just those pertinent to engines.
Impact on Career and Students
This research has had a tremendous effect on the career of one of the principle investigators by supporting his primary research, and has had a big influence on a number of undergraduate students who were able to obtain intensive research experiences. A total of fourteen undergraduate students have participated in our film evaporation research, though not all have been supported by the grant. Five of the students have graduated and at least four of these students have continued their education in graduate school. Additionally, the research has had a big impact on Trinity University's Engineering Science Department. The research program supported by the ACS PRF was the first in the Engineering Science Department in a long time to provide undergraduate summer research opportunities, and has acted as a catalyst to promote an increased level of Departmental research activity.
Research Progress
During
the 2007-2008 reporting period, we focused on two studies: 1) an investigation
of the relative importance of gas phase diffusion and natural convection on the
evaporation rates of films in a quiescent environment, and 2) the importance of
liquid phase diffusion on the evaporation rates of films composed of a
bi-component mixture.
Gas Phase Diffusion and Natural Convection
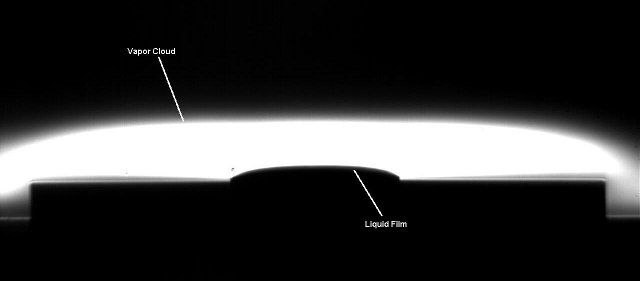
Almost immediately after a liquid film is generated, a layer of vapor forms above the film. Since the vapor is heavier than the surrounding air, the tendency of the layer to grow vertically out from the film due to diffusion is constrained by a buoyancy force which acts to push the vapor layer down and radially outward. Through the use of schlieren imaging, which is an optical technique to visualize the transparent vapors, and gravimetric analysis, the effects of natural convection of the vapors on the evaporation rate of hydrocarbons having a wide range of volatilities were studied.
Figure 1 is a schlieren image of an evaporating pentane film and shows the vapor cloud, which is the white region located directly above the film, flowing radially away from the liquid film. As a means of varying the influence of buoyancy, the geometry surrounding the film was varied. Experiments were conducted with the film level with a surrounding horizontal surface (as shown in Fig. 1), with the film raised above the surface on a pedestal, and with the film recessed below the horizontal surface in a cavity or well.
Figure 1. Schlieren image showing the vapor cloud above
an evaporating pentane film.
The
experiments clearly indicate that both diffusion and buoyancy-induced convection
can have significant effects on the evaporation rate of a liquid film,
depending on the surrounding geometry.
However, we have not quantified the relative contributions of the two
transport mechanisms. In an attempt to quantify the rates of vapor transport by
diffusion and convection, we have begun to develop experiments to measure the
vapor concentration distribution as a function of position above the liquid
film using infrared spectroscopy coupled with computer tomography. Liquid
Phase Diffusion Our
study of liquid phase diffusion involves measuring the instantaneous
evaporation rate of mixtures of a volatile and non-volatile component. If the liquid remains uniformly mixed then
the evaporation rate would be directly proportional to the concentration of the
volatile component in the film. If
diffusion within the film is slow compared to the rate of evaporation, then the
concentration of the volatile component at the surface of the film may be lower
than the overall concentration in the film.
In this case, the slow rate of diffusion can limit the evaporation rate,
as appears to have occurred in our preliminary measurements.
Future Research We
continue to work to elucidate the roles of the various transport processes
involved in hydrocarbon film evaporation.
In the future, we intend to focus on the evaporation of mixtures and on
the effects of increased temperature and pressure, using a specially-designed
temperature-controlled pressure chamber.