

47118-G6
Control of Molecular Organo-metallics Conductance via Changes in the Ligation Scheme
The research lead by the PI has made progress in the area of the proposed studies of molecular and nano-scale conductance. In particular, schemes based on both chemical and physical means to control molecular conductances have been investigated by the PI's research group. The calculations provide insights at the atomic level to complement experimental observations or predictions for stimulating additional experiments.[1,2,3,4,5,6,7]
The studies of conductance of molecular systems involving changes in the metal ligation scheme[1,3,5] bear the most relevance to the funded research program. These studies include conductance switching of an iron-porphyrin system,[1] the interplay of an appropriate peptide molecule ligating a metal ion,[3] and the analysis of a device based on Co-terpyridine complexation reaction within a confined gap.[5] We next describe in more detail the studies of relevance for the PRF funded grant period.
The PI's research group studied an experiment led by Prof. Tao,[8] in which a strong conductance enhancement was associated with metal ions that reacted with a specific peptide-based self-assembled surface monolayer. Other ion-peptide combinations resulted in a minimal conductance response. The reaction associated with this response is illustrated in figure 1i. To explain these measurements, the PI's research suggested several intriguing details with regard to the transport mechanism. The computational models point to ligation-induced carbonyl-gold interactions as responsible for the (selectively) observed enhanced conductance.[3] The computational cluster models and calculated electronic transport functions are depicted in Figure 1 parts ii and iii, respectively, and are further discussed in the paper[3].
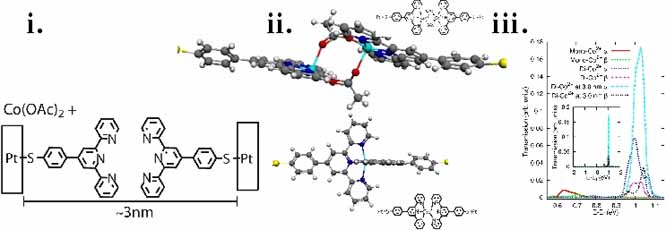
Another high-profile experiment was also successfully simulated by the PI's research group.[5] In the experiment led by Prof. Nuckolls, Co(II) ions, by a combined means of lithography and chemical self-assembly, were used to assemble molecular devices that bridge conjugated organic systems.[9,10] Structure-function relationships, which had not been considered before, are indicated by the PI's calculations to explain these experiments.[5] The results, which are illustrated in Figure 2 and in the submitted research-nugget, provide atomic scale characterization required for interpreting experimental observations. We also note that the research report related to the SUMR program, which is also submitted in an attached document, lists the contributions of a student involved in a project that is built on our initial analysis of the Co-terpyridine complex-enabled conductance.
The PI also studied non-chemical means to tune conductances. For example, structure-function relationships affecting the functionality of field-effect transistors were analyzed by a series of papers.[4,6,11] The group's studies point to a symmetry-breaking effect, that depends on certain contact orientation features to determine the gating response.[4] In follow-up studies, which were funded by the PRFG grant, the original report is complemented by further analyzing the possibility of enhancing the gating response by chemical substitutions [6] and the delicate dependence of the gating response on the geometrical features of the contacts,[11] which wil be described in the next report. In another related study pursued during this funding period the conductance changes of Pd wires due to hydrogen molecules were analyzed[7]. The calculations suggest that such systems have the potential to serve as highly sensetive hydrogen sensors, an application of importance to the emerging hydrogen economy-based fuel technology.
Figure 1:
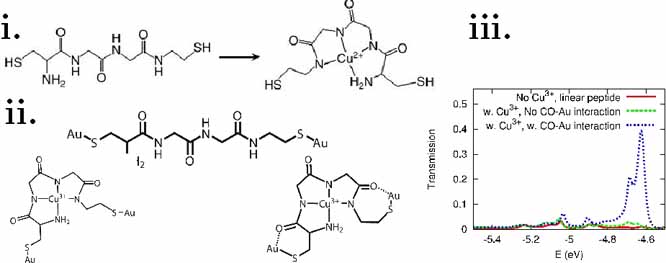
Figure 2:
References
- [1]
-
Chen, Y., Prociuk, A., Perrine, T. and Dunietz, B. D. Phys. Rev. B , 74, (2006), 245320.
- [2]
- Das, M. and Dunietz, B. D. J. Phys. Chem. C , 111, (2007), 1535-1540.
- [3]
- Perrine, T. and Dunietz, B. .D. Nanotechnolgy , 18, (2007), 424003.
- [4]
- Perrine, T. and Dunietz, B. D. Phys. Rev. B , 75, (2007), 195319.
- [5]
- Perrine, T. and Dunietz, B. D. J. Phys. Chem. A , 112, (2008), 2043.
- [6]
- Perrine, T. M., Smith, R. G., Marsh, C. and Dunietz, B. D. J. Chem. Phys. , 128, (2008), 154706.
- [7]
- Zhao, Z. and D., Dunietz B. J. Chem. Phys. , 129, (2008), 024702.
- [8]
- Xiao, X., Xu, B. and Tao, N. Angew. Chem. Int. Ed. , 45, (2004), 6148-6152.