45134-B7
Competition between Mesogens in Pyridone-Based Supramolecular Calamitic, Banana, and Discotic Liquid Crystals
In the first year, Justin Kumpfer worked on the synthesis of pyridone-based esters. It was found that while the substitution chemistry of pyridones was difficult, the species were capable of reacting through nucleophilic acyl substitutions. Metal-based coupling reactions proved problematic, as the pyridine functionality chelated the metal from the catalyst, rendering the reaction inert. These results provided the groundwork for the rest of the project.
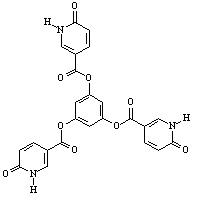
Figure 1: Pyridone Derivatives |
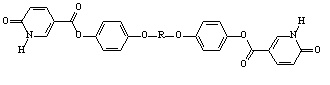 In
the second year, David Witte synthesized a series of bis-functionalized
pyridone terminated esters. One set of supramolecular esters, seen in Figure 1,
is based on alkylated hydroquinone derivatives. The R
group in these molecules included a decane chain, as
well as a series of oligomeric (3,4,5)
ethyleneoxy chains. These can be seen in Figure 1. Supramolecular polymers produced flexible,
long-lived fibers pulled from the melt, but
In
the second year, David Witte synthesized a series of bis-functionalized
pyridone terminated esters. One set of supramolecular esters, seen in Figure 1,
is based on alkylated hydroquinone derivatives. The R
group in these molecules included a decane chain, as
well as a series of oligomeric (3,4,5)
ethyleneoxy chains. These can be seen in Figure 1. Supramolecular polymers produced flexible,
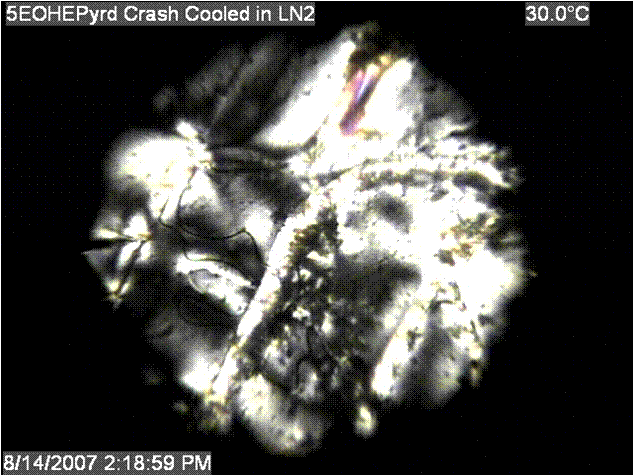
long-lived fibers pulled from the melt, but Figure 2: Frozen Nematic of a Pyridone Mesogen |
 interestingly produced only a frustrated
nematic phase observable only upon crash cooling the isotropic melt in liquid
nitrogen. It is believed that the nematic phase could only be captured in this
dramatic fashion because the overall structure of the assembled pyridone
species would be too irregular to effectively form a mesogenic phase. Figure 2
shows the frozen nematic phase obtained from one of these complexes. Results of
this work were presented at the Boston ACS meeting in August of 2007. A
manuscript is in development to be submitted to Liquid Crystals. This paper
will have three undergraduate authors.
interestingly produced only a frustrated
nematic phase observable only upon crash cooling the isotropic melt in liquid
nitrogen. It is believed that the nematic phase could only be captured in this
dramatic fashion because the overall structure of the assembled pyridone
species would be too irregular to effectively form a mesogenic phase. Figure 2
shows the frozen nematic phase obtained from one of these complexes. Results of
this work were presented at the Boston ACS meeting in August of 2007. A
manuscript is in development to be submitted to Liquid Crystals. This paper
will have three undergraduate authors.
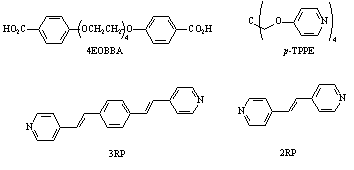
A side project funded by this grant was carried out by Jason Greuel involving the investigation of the liquid crystalline properties of supramolecular networks. In this we report the formation of a series of mesogenic networks with increasing netpoint inclusion. The mesogenic portions of these systems arise from a hydrogen bonded interaction between a bisbenzoic acid (tetraethyleneglycoxy-bis-4-benzoic acid, 4EOBBA) and a pair of rigid bispyridyl systems of increasing rod length (1,2-bis(4-pyridyl) ethylene, smaller rod: 2RP; 4,4'-(p-phenylenedi-1,2-ethenediyl)bispyridyl, longer rod, 3RP). A tetrafunctionalized non-mesogenic networking agent (tetrakis-4-pyrixyloxy methane, p-TPPE) is used to introduce a network and disrupt liquid crystallinity. All the materials uses in this study are outlined in Figure 3. The networking agent was added in increasing concentrations to
the system. All the networks display mesogenic characteristics at a surprisingly high network concentration, up to 25%. The 3RP-containing networks exhibit higher clearing and mesophase reformation temperatures than the shorter systems, and displayed mesophase characteristics at higher inclusion of disruption. It would seem that the lability of the hydrogen bond would allow for molecular reorganization to form the more stable mesogenic phase to form at higher concentrations than would normally be seen in covalent analogs.
Figure 4: Smectic Phase Observed From 5% 2RP Network
|
 Optical micrographs for this study can be seen
in Figure 4. A further study involved annealing these networks in the mesophase
and observing a premature onset of
Optical micrographs for this study can be seen
in Figure 4. A further study involved annealing these networks in the mesophase
and observing a premature onset of Figure 3: Materials for Network Study |
 crystallization. This onset rose to higher
and higher temperatures with increasing netpoint
inclusion. This phenomenon was not observed in small molecule covalent or
supramolecular liquid crystals. The first study has produced a paper which will
soon be submitted to Liquid Crystals, and the second manuscript is in
development. Both will have two undergraduate authors.
crystallization. This onset rose to higher
and higher temperatures with increasing netpoint
inclusion. This phenomenon was not observed in small molecule covalent or
supramolecular liquid crystals. The first study has produced a paper which will
soon be submitted to Liquid Crystals, and the second manuscript is in
development. Both will have two undergraduate authors.
Further work will include investigating non-calamitic mesogen types- specifically discotic mesogens. We will be investigating the creation of disc-shaped liquid crystalline architectures utilizing the double hydrogen bonding moieties of the pyridone group. This will stem from esters formed from the reaction of phloroglucinol and 6-hydroxynicotinic acid, as seen in Figure 5. These will then be complexed with small molecule analogs of the system seen in Figure 1 to determine if discotic mesophases can be formed.
|