46669-G3
Zirconium-Mediated Bond-Forming Catalysis
Part 1. Impact of PRF support
Support from the PRF has had a great impact on my career very rapidly. As is the intention of this starter-type grant, I was able to use some preliminary results from PRF-funded research in an application for NSF funding. The PRF-funded results were clearly influential as I was granted a CAREER Award from the NSF on May 1, 2008. Furthermore, PRF support has resulted in printed two publications, two more in press, and additional manuscripts forthcoming in the new grant period. It has been a productive grant for my research group, and PRF support has been key in establishing significant momentum for my research program.
I have funded several students with PRF funds. These funds released the students from teaching responsibilities to pursue the goals of this project fully. This time has been a great boon in their training and the resulting publications are a testament to their productivity. Moreover, time where a student can be fully dedicated to research is key in their development as scientists. One undergraduate student was funded with this grant, and she is currently applying to gratuate programs in chemistry.
Part 2. Research progress
Initial efforts into tripodal phosphorus ligand preparation has been stymied by synthetic difficulties. In our hands, ligand preparations gave limited reproducible results. The difficult synthesis suggests that these ligands are not currently suited for catalytic studies. In an effort to make more significant research progress, I decided to shift our efforts toward catalytic chemistry, the heart of the proposed research, using more readily prepared ligands.
Triamidoamine ligands were selected for our initial catalytic reactions because of the similarity to the desired phosphido ligands. Furthermore, we were having successes in discovering catalytic reactions of triamidoamine zirconium complexes. In particular, we have observed catalytic dehydrocoupling and heterodehydrocoupling of phosphines with high selectivity.
Initial studies focused on bond-forming catalysis involving arsenic. We quickly discovered that our zirconium triamidoamine complexes are competent for the catalytic dehydrocoupling of arsines to form As–As bonds with liberation of hydrogen. PRF support allowed us to discover the first dehydrocoupling catalyst for arsines. As we investigated the mechanism of this transformation, things became more interesting. Secondary arsines, in particular diphenylarsine, were dehydrocoupled by a mechanism that was very similar to that seen for phosphines. In these reactions, our zirconium complexes readily dehydrocoupled diphenylarsine to tetraphenyldiarsene in high yields. Evidence through kinetic investigations supported a mechanism consistent with sigma-bond metathesis steps for As–As bond formation.
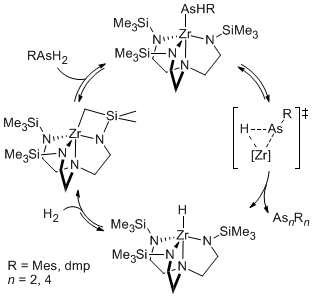
However, for sterically encumbered primary arsines, the observed products, including diarsenes (RAs=AsR) were not consistent with a sigma-bond metathesis mechanism. For instance, the dehydrocoupling of dmpAsH2 (dmp = 2,6-mesitylphenyl) gave the diarsene product, dmpAs=Asdmp. Likewise, catalytic dehydrocoupling of mesitylarsine afforded the arsacycle (MesAs)4. efforts to improve the selectivity for the diarsene gave indirect evidence for low-valent arsenic. Addition of trimethylphosphine to the catalysis provided a much higher yield of diarsene. The arsa-Wittig reagent, dmpAs=PMe3, has been reported, and it decomposes to the diarsene. Though we did not observe this complex, we were encouraged to consider lw-valent arsenic based on these results. Kinetic studies revealed evidence consistent with alpha elimination or the extrusion of a low-valent fragment. In particular, we noted a first-order dependence on the decomposition of zirconium arsenido complexes to the tetraarsine product. Interestingly, we were also able to obtain structural data for these zirconium arsenido complexes to provide insight into the rare Zr–As linkage. The observation of alpha-arsinidene elimination is a substantial advance because this is the first instance of alpha-elimination catalysis for arsenic, and in previous reports, alpha elimination is a process associated with heavier main group elements. Our observations suggest that this kind of catalysis can be promoted for lighter elements and give access to new synthetic methodologies. A proposed catalytic cycle for the dehydrocoupling of primary arsines based on alpha-elimination is shown below.
Finally, these same triamidoamine zirconium catalysts were demonstrated to engage in hydroarsination of terminal alkynes—the only early metal complexes known the catalyze this As–C bond forming reaction. We have only limited mechanistic data on this reaction, but stoichiometric studies with polar small molecules suggest that the hydroarsination is proceeding via insertion of the alkyne into the Zr–As bond.
As can be seen, we have made substantial progress as a result of PRF support and opened new avenues of research that will be pursued in the coming grant period.