

42853-AC1
Asymmetric Synthesis via Organocopper Chemistry
The aim of this project is to develop efficient asymmetric synthesis strategies involving organocopper chemistry employing either enantioenriched cuprates or enantioenriched electrophiles (e.g., propargyl and allylic substrates) or both at the same time. We have found that 4-pyridones 1 undergo 1,4-conjugate addition reactions with organozinc and cuprate reagents to afford 5,6-dihydropyridin-4-ones 2 in good to excellent yields, and have now found conditions to effect the conjugate addition of ethylcuprates with good enantioselectivities. The scope and limitations of this enantioselective approached to dihydropyridinones is under investigation.
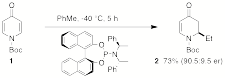
Work
has also been directed toward controlling the chemo-, regio-, and
stereoselectivity in the reactions of organocuprates with
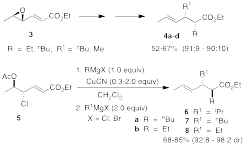
gamma,delta-heteroatom substituted alpha,beta-enoates. Sequential cuprate mediated allylic
substitutions on epoxyenoates 3 affords
2,3-disubstituted-4-hexenoates 4
in modest yields and with excellent syn-diastereoselectivity. One-pot tandem cuprate mediated allylic
substitution reactions on ethyl 4-halo-5-acetoxy-2,3-hexenoate (e.g., 5) gave excellent chemo-, regio-, and
stereoselectivity to afford anti-2,3-disubstituted-4-hexenoates 6a-b-8a-b. Optimal reaction conditions involved the
use of Grignard reagents, CH2Cl2, and 0.30 equivalents of
CuCN.
These developments reveal that
excellent regio- and sterocontrol can be achieved in these sequential or tandem
allylic substitution reactions and efforts are underway to broaden this strategy
for the synthesis of O and N-heterocycles.
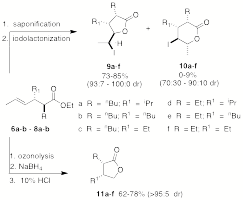
In this regard, we have shown that anti-2,3-disubstituted-4-hexenoates 6a-b-8a-b can be readily converted into iodolactones 9a-f in
good yields and with excellent
diastereoselectivities contaminated with only small amounts of the
delta-lactones 10a-f. The
minor delta-lactones can be easily removed by column chromatography. The synthetic methodology provides for
a five step synthesis of gamma-lactones with the stereocontrolled formation of
4-contiguous stereogenic centers.
A three step procedure converts enoates 6a-b-8a-b
into the cis-3,5-disubstituted-gamma-lactones 11a-f
that are difficult to access by other routes.
Support
for this project has enabled us to achieve proof of concept for utilizing tandem
allylic organocopper substitution reactions for generating contiguous
stereogenic centers in a stereocontrolled fashion. We anticipate extending this work to the use of tandem
sequential allylic substitutions and 1,4-additions mediated by both palladium
and copper reagents. This support
by ACS-PRF has enabled one student to nearly complete his Ph.D. work and
another to make substantial progress toward a degree.