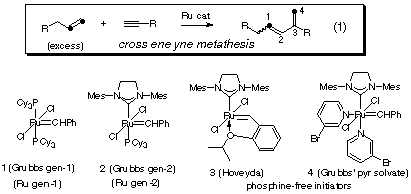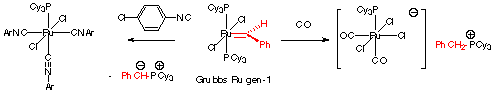44202-AC1
Studies on the Mechanism of Enyne Metathesis
The collaborative ACS-PRF sponsored research is directed towards the study of the enyne metathesis reaction mechanism. The enyne metathesis and the Grubbs carbenes that are commonly used are illustrated in Scheme 1. The primary research activity during year one was devoted to a full investigation of the ligand-promoted insertion for a variety of ruthenium carbene complexes and the development of a practical ruthenium carbene clean-up procedure for metathesis. This insertion was proposed as a way to stop metathesis reactions for kinetic analysis to study reaction mechanism, but it has evolved into a practical procedure to both stop a metathesis reaction and to facilitate purification of the organic products. In year two, we have used this reaction development to stop first generation catalysis (one paper published) and to expand the reaction scope in enyne metathesis, publishing three new methodology papers. The kinetic work in the first generation Grubbs catalyzed enyne metathesis is ongoing and no data has yet been published.
Scheme 1. Ene-yne Metathesis and the Grubbs Carbenes
Ruthenium
Clean-Up Procedure In
the clean-up procedure, there has been continued study in developing new
isocyanides to assist in the quenching and clean up of metathesis reactions.
These studies are not complete and have not been published. The products
arising from CO and isocyanide quench in the Grubbs Ru gen-1 system have been
elucidated (Scheme 2). Scheme
2. Ligand-Promoted Insertion in the
First Generation Grubbs Complex
Mechanism
of Ene-Yne Metathesis Mechanistic
studies have continued in this second year as the insertion chemistry was
wrapped up and published. We have
now developed the necessary quench procedure for the full range of Grubbs'
ruthenium carbenes including the first and second generation ligand
environments. As a result, we have turned our attention in year two towards
enyne metathesis using the first generation Grubbs carbene complex. The first
generation complex is still widely used in organic synthesis for metathesis
involving ethylene. We are now obtaining reaction kinetics using the first
generation complex and expect to have publishable results by the end of the
calendar year. In addition, the fast quenching procedure is being used to study
rapid enyne metathesis employing very reactive phosphine-free variations of the
Grubbs catalyst. We will interpret these data in terms of our ongoing synthetic
studies in ene-yne metathesis, and collaterally use this knowledge to develop
new applications of the ene-yne metathesis. Enyne
Metathesis Methods Development In year two, we have also employed our mechanistic
knowledge of the enyne metathesis to develop three new reactions. First, we
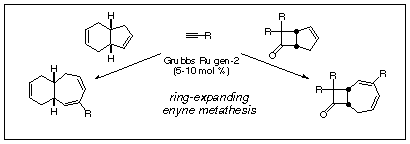
have reported our preliminary work on the ring expansion chemistry, which we
call "ring-expanding enyne metathesis" which should prove broadly useful for
the preparation of medium rings (Scheme 3). Second, we have developed the cross
enyne metathesis between unsaturated alcohols and alkynes, despite concerns
regarding carbene catalyst decomposition in the presence of alcohols. It should
be noted that the influence of proximal functional groups like alcohols is not
well understood in metathesis chemistry. Most recently, we have driven geminal
alkenes to participate in the cross enyne metathesis using ring strain. These
studies have collectively benefited from mechanistic knowledge obtained under
the ACS PRF grant. Scheme
3. Ring Expanding Enyne Metathesis
In conclusion, the second year of this PRF grant has
resulted in a relatively advanced understanding of fundamental reactivity of
the Grubbs ruthenium carbenes in the presence of strong donor ligands. This
information is vital for a successful kinetic study for fast enyne metatheses
and those involving internal alkynes where IR cannot be used to follow the
reaction kinetics. Last, our evolving mechanistic knowledge has resulted in the
development of synthetic methods employing enyne metathesis for ring expanding
reactions and for difficult cross enyne metathesis. Last, we are grateful to
the ACS-PRF fund for support of this research project, which has involved five
graduate students and four undergraduate students during the past two years.