46982-AC3
New Robust Tridentate N-Heterocyclic Carbene Ligands for C-H Activation
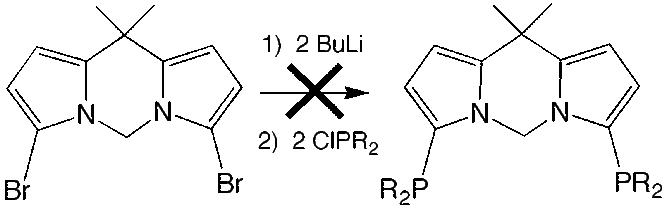
During the past 12 months, much progress has been made. The synthesis of the original PCP ligand has been improved and made more efficient. While it is still a multi-step procedure, each of the steps has been optimized to give reproducibly high yields. We have also examined the synthesis the proposed bis(pyrrole) ligand system. While the organic framework could be assembled, the introduction of the flanking phosphine units could not be achieved under any circumstances.
For this reason, we have
abandoned this alternative ligand system to focus entirely on the original PCP
ligand. We
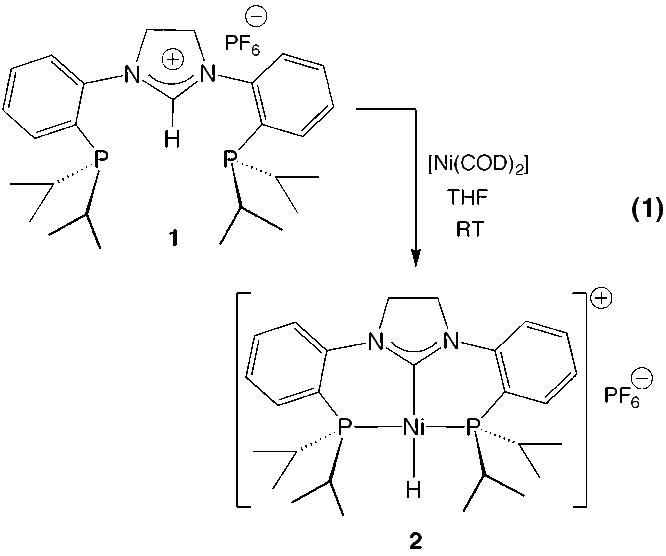
have discovered that the ligand system as the imidazolidinium salt, [(PCP)H]PF6
can easily be installed on the group 10 metals to generate the corresponding
hydride complexes, [(PCP)MH]PF6, where M = Ni, Pd and Pt. Equation 1
shows the preparation of the Ni-hydride:
All of the group 10
complexes have been characterized fully by NMR spectroscopy, elemental analyses
and by X-ray crystallography. The nickel hydride undergoes a fascinating
reaction with ethylene to generate a new carbon-carbon bond at the carbene
carbon. By following this reaction
using deuterium labeling, we were able to propose that the process first
involves insertion of ethylene into the Ni-H bond followed by a reductive
elimination of the Ni-ethyl and the Ni-carbene. In an effort to shed light on the specifics of this process,
we initiated a collaboration with Professor Jennifer C. Green at the Inorganic
Chemistry Laboratory at Oxford University. Using DFT calculations, she was able to provide us with a
reasonable clue as to what the transition state looks like. In addition, her calculations showed
that the isopropyl groups on the phosphine flanks of the tridentate ligand
exert a profound steric effect on this process. The significance of this study is that is shows another way
that these N-heterocyclic carbene ligands can act non-innocently in a chemical
transformation. We
have also examined the introduction of this PCP ligand system onto rhodium and
iridium, which are central to the proposal in examining C-H activation processes. This has been incredibly successful as
we have been able to prepare a variety of useful precursors, which will serve
us well in the following year. Our
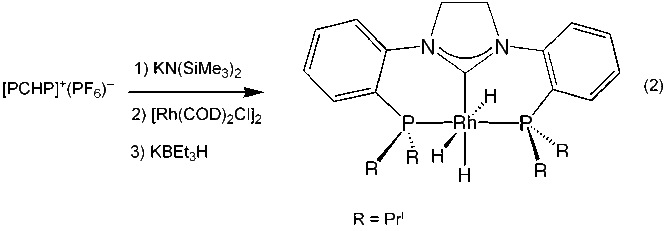
initial attempts to use the same imidazolidinium salt that worked so well with
the group 10 metals failed for the heavier members of group 9 (Rh(I) and Ir(I)). However, deprotonation of the
salt with KN(SiMe3)2 generates the bisphosphinecarbene
that can be directly added the 1,5-cyclooctadiene dimers, [M(COD)Cl]2,
to generate the square planar derivatives (PCP)MCl, where M= Rh, and Ir. Subsequent reaction of the rhodium
complex (PCP)RhCl with KBEt3H generates the corresponding hydride
complex (PCP)RhH, which we have characterized fully; this is shown in equation
2.
This complex reacts with H2
to generate a trihydride, which can be characterized by NMR spectroscopy. We are now looking at the thermal
reactions of theses systems with a variety of alkanes to examine their ability
to activate and functionalize hydrocarbons, which will be of interest as a new
way to functionalize petroleum products.
Future work will focus on group 9 as well as group 8 metals, in
particular Ru and Os.