46419-AC9
Mechanistic Understanding of Wettability Alteration in Fractured Carbonate Reservoirs
The goal of this proposal is to develop a mechanistic understanding of the effect of potential determining ions, surfactants, oil composition, and temperature on the wettability of carbonate reservoir minerals. This knowledge can be used to alter wettability of initially oil-wet fractured carbonate reservoirs to recover oil from the matrix blocks. The results of wettability studies are reported here. The effect of temperature on wettability and core-scale studies are under progress.
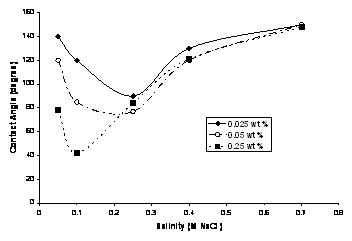
Wettability alteration of a calcite plate can depend on parameters like brine salinity, oil composition, type of surfactant, concentration of surfactant, and temperature. Most of these experiments are done with a model oil (1.5 wt% cyclohexanepentanoic acid in n-decane). Figure 1 shows the dependence of the final contact angle on salinity at a fixed Alf-38 concentration. Sodium Chloride (NaCl) was used to change the brine salinity keeping sodium carbonate concentration fixed at 0.25 wt%. Na2CO3 was added to keep the brine pH above the point of zero charge of calcite. As pH increases, the calcite surface charge changes from positive to negative as it crosses its point of zero charge (PZC). Crossing PZC reduces the adsorption of anionic surfactants on carbonate rocks. The PZC for limestone is around 9.2 and the PZC for dolomite is about 7.4. Figure 1 shows that for a given Alf-38 concentration, the contact angle decreases and then increases with the increase in salinity. The salinity at which the contact angle is the minimum is termed as the optimal salinity for wettability.
Figure 1: Effect of salinity on contact angle at a
fixed 0.25 wt% Na2CO3 for varying Alf-38 concentration
(using model oil) Earlier studies have
shown that for a given surfactant concentration there exits an optimal salinity
which gives a minimum IFT. To understand
the relation between optimal salinity for a given surfactant concentration for wettability and optimal salinity for IFT, we measured both
contact angles and IFT with the model oil for 0.1 wt % Alf-38 at a fixed 0.25
wt% Na2CO3 and variable NaCl
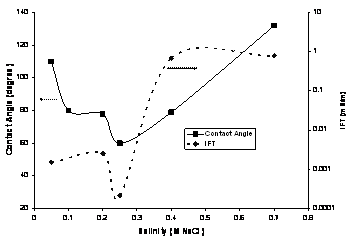
concentrations, as shown in Figure 2.
The water to oil ratio (WOR) ratio was one in IFT experiments and very
large (>100) in contact angle experiments. The results show that the optimal
salinity for wettability and IFT coincides, at about
0.25 M NaCl. This result also supports the argument
that optimal values for IFT and wettability occur at a salinity where surfactant has equal affinity for oil and
aqueous phase.
Figure 2: Contact angle and IFT
values for 0.1 wt% Alf-38 at varying salinity with 0.25 wt% Na2CO3
(using model oil) We investigated the effect of
divalent ions like SO42- and Ca2+ ions on the wettability of a calcite plate with the model oil without
any surfactant. The equivalent monovalent ion
concentration is kept constant at 0.576 moles per liter in all the brines. The
base case magnesium, calcium and sulfate concentrations were taken as 0.045,
0.013 and 0.025 M, respectively. Brine MgCa2S has twice the base case sulfate;
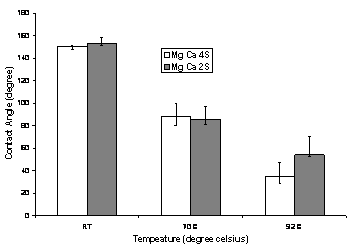
brine MgCa4S has four times the base case sulfate. Figure 3 shows the effect of
sulfate ions in the presence of Mg2+ and Ca2+ ions on wettability of calcite with temperature. At room
temperature, brines MgCa2S and MgCa4S could not alter the wettability
with advancing contact angle ~150°. At 70°C, these brines alter the wettability of the plates to intermediate wet condition
with contact angle values ~86°. At 92°C, MgCa4S was found to have advancing
contact angle of 35° and MgCa2S had 54°, thus rendering the calcite surface to
water-wet conditions. The SO42- ions at the higher
temperature tend to desorb cyclohexanepentanoic
acid from the solid surface and occupy the sites freed.
Figure 3: Effect of
temperature on contact angle for brines MgCa4S and MgCa2S (using model oil;
RT=room temperature)