47286-GB5
Density Functional Theory and Car-Parrinello Molecular Dynamics Simulations of Ethanol Electro-oxidation Reaction on Bi- and Trimetallic Nanoparticles
To gain a good understanding about the mechanism for PtSn alloy to catalyze ethanol electro-oxidation reaction, adsorption and decompositions of H2O and ethanol over PtnM clusters have been systematically investigated. CPMD simulations for the relevant system are in progress.
1. H2O adsorption and decomposition over PtnM clusters (n=2, 3 and 9; M=Pt, Sn, Ru)
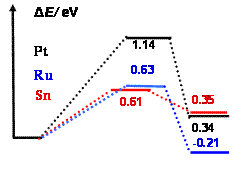
The decompositions of adsorbed water over the clusters Pt3, Pt2Sn and Pt2Ru, were found to have two pathways: atop and bridge decomposition. For the Pt3, atop decomposition has lower energy barrier than the bridge one; while for the adsorptions over Sn and Ru sites of Pt2M the energy barriers for bridge decompositions are lower than atop ones. The kinetic favorable pathways for water decomposition over Pt2M in Figure 1a, shows that decompositions of H2O over Sn and Ru sites are kinetically more favorable than Pt site. The adsorption of water on the Sn site of Pt3Sn is quite weak with O-Sn distance of 2.84 Å and only -0.20 eV of adsorption energy, and failed to obtain the decomposition transition state for the Sn site adsorption.
Figure 1. Potential
energy profiles for the adsorbed water decomposition over Pt2M
(M=Pt, Sn and Ru) (a, left) and Pt9M (b, right).
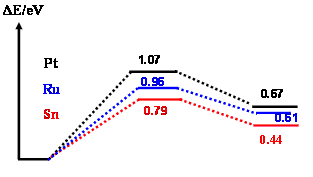
To eliminate the edge effect, a two
layer cluster, Pt7Pt3 has been used to model Pt-M alloy
surface,1 and the central atom on the surface has
been replaced by alloyed atoms. To provide structural information and accurate
barrier, the transition states are directly searched for rather than estimated by the UBI-QEP formula as in Ref. 1. The
binding energy of H2O on Pt10 was predicted to be -0.26eV
in this work, which is rather close to the DFT calculation of water on Pt(111)
surface, -0.29eV.2 It shows that
the central Sn has a quite stronger adsorption to H2O (Ead, -0.80eV), but similar to
Pt3Sn water only weakly binds to Sn as Sn locates on the edge. According
to Figure 1b, it is interesting to find that thermodynamically and kinetically the
decomposition of the adsorbed water on Sn site is more favorable than the
decomposition on Pt and Ru sites. This result supports the assumption of the bi-functional
mechanism, where Sn site facilitates the dissociation of H2O.
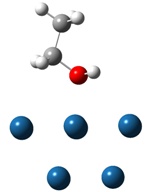
2. Adsorption and Dissociation of Ethanol over PtnM
clusters Two major adsorptions of ethanol via
hydroxyl and methylene/methyl groups, and subsequent decompositions are
investigated (shown in Figure 2). The former is more stable than the latter
over Ptn (n=3,4 and 9) by 0.3-0.4eV, and we fail to obtain the methylene
adsorption over PtnSn clusters.
Figure 2. Two major adsorptions of
ethanol on Pt10
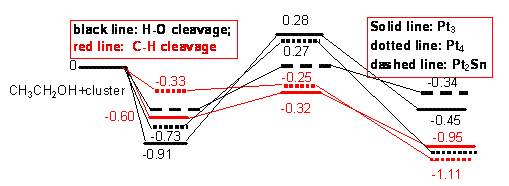
Figure 3. The cleavage mechanisms of ethanol through OH and CH2
adsorptions over Pt3, Pt2Sn and Pt4.
According to Figure 3, in spite of stronger
adsorption the energy barrier for the cleavage of the adsorbed OH is
significantly higher than that for the homolytic decomposition of the adsorbed CH2,
and thermodynamically is also less favorable. Although the two
possible mechanisms via either O or CH2 adsorption have been
proposed, the current investigation shows that the decomposition resulting from
the adsorption of CH2 is more likely to occur preferentially.
Relative to individuals, the C-H bond cleavage even exhibits negative energy
barriers. Since ethanol adsorption on Sn site of PtnSn through CH2
is unstable, the corresponding cleavage seems unlikely. Thus, the results
support the assumed bi-functional mechanism from another viewpoint that ethanol
decomposition occurs over Pt site.
3. Impact on my
career and on the students In
the “Computational Chemistry” (Fall, 2007) course, 4 hours were spent on molecular
modeling and simulations of fuel cell systems. The projects of two chemistry
seniors, Jessica Holiday and Natalie Redmon, are “Quantum chemistry studies on
the ethanol oxidation reaction over metal clusters”. They also got opportunity
to use the high performance Linux cluster provided by the NCSA at the References: