44891-B3
Preparation, Characterization, Film Fabrication, and Photoluminescence of Metal-Organic Networks of Copper(I) Cyanide
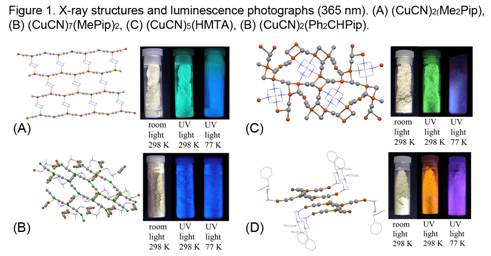
The major finding from last year that copper(I) cyanide forms diamine and diimine networks has been further confirmed and expanded. The networks of CuCN with N-substituted piperazines (e.g., Me2Pip = N,N′-dimethylpiperazine, MePip = N-methylpiperazine, EtPip = N-ethylpiperazine, Ph2CHPip = N-diphenylmethylpiperazine) and hexamethylenetetramine (HMTA), among others, have been studied. These diamine and tetramine ligands (B) produce stable, highly luminescent (CuCN)n(B) networks self-assembled in single-step, high-yield, aqueous reflux reactions of CuCN and B in the presence of KCN.
A common structural theme is the formation of (CuCN)2B hexagonal honeycomb 63 networks, which consist of tiled Cu6(CN)4B2 units as a result of bridging B and mu2-cyano groups. This motif is very common with piperazine networks of CuCN, such as (CuCN)2(Me2Pip), Figure 1A. The 63 network pattern is also found threaded with additional CuCN chains in relatively copper-rich phases, such as (CuCN)7(B)2 = 2[(CuCN)2B]•3CuCN (B = MePip, EtPip). In Figure 1B the 63 network is viewed edge-on and is illustrated with its Cu atoms colored orange; the threaded CuCN chains are shown with Cu atoms colored green. (In all X-ray figures organic ligands are shown as wireframes and all cyano C/N atoms are colored grey to reflect site disorder.) CuCN-threaded networks are also noted for (CuCN)4(B) = [(CuCN)2(MePip)]•2CuCN (B = Me2Pip, Et2Pip). Non 63 network motifs are seen for (CuCN)n(HMTA) (n = 1.5, 2.5, 5) and (CuCN)2(Ph2CHPip), as illustrated in Figures 1C and 1D. The HMTA compounds show extensive mu3-cyano bridging and the Ph2CHPip compound reveals a CuCN chain uniquely decorated with -CN-B units.
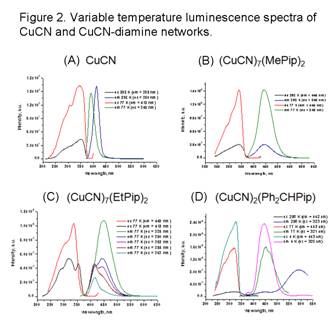
The new copper(I) cyanide networks show interesting spectroscopic
behavior. CuCN itself is colorless, absorbing in the
UV (see Figure 2A), but luminesces intensely at the border of the UV and
visible. The new diamine and tetramine networks are more-or-less white but in
many cases are highly luminescent. This phenomenon is currently being investigated
collaboratively by the P.I. with Prof. Howard Patterson (University of Maine,
variable temperature spectroscopy) and Prof. Craig Bayse
(Old Dominion University, computational analysis). The major findings are:
1) 2) 3) 4)
Research has
been initiated on the luminescence of monoamine (L) adducts of CuCN. It has been found that addition of small amounts of
liquid or vapor-phase amine to solid CuCN causes
luminescence emission to shift from the near UV value of 392 nm characteristic
of CuCN to wavelengths well into the visible. Figure
3 reveals the varied effects of a variety of liquid amines on the luminescence
emission of CuCN. As shown in Figure 3, slight
chemical changes can produce the significantly different emission colors, e.g., green for CuCN/piperidine
(Figure 3G), yellow for CuCN/N-methylpiperidine (3H)
and pink for CuCN/N-ethylpiperidine (3I). The results
of thermogravimetry and X-ray powder diffraction have
indicated the following facts:
1) 2) 3)
These
findings are of interest with regard to potential chemical sensors for volatile
organics in the environment. Based on the above results, a putative “sniffing”
device based on CuCN would be able to distinguish
closely related N-alkylpiperidines. It would also be
able to distinguish 2-, 3-, and 4-methylpyridine.