
Back to Table of Contents
40904-GB1
New Applications of Cobalt-Alkyne Complexes in Organic Synthesis
Kevin M. Shea, Smith College
The final report for this PRF
award will describe the progress we have made on two projects outlined in
earlier reports and one project that we initiated over the past year. The theme of our PRF-funded research continues
to be the development of new synthetic applications of cobalt-alkyne complexes
in Diels-Alder reactions. Specifically,
we continued our investigations into the synthesis and reactivity of dienes
adjacent to cobalt-complexed alkynes, and we developed cationic Diels-Alder
dienes stabilized by cobalt-alkyne complexes.
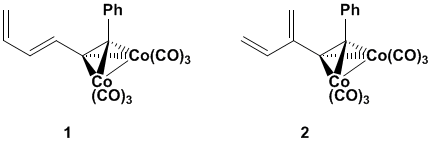
Earlier reports described our
strategies for the synthesis of dienes 1
and 2, which we hoped would be
highly reactive in Diels-Alder reactions.
As previously summarized, diene 1
could be prepared quickly and efficiently; however, it was unreactive with
nearly all dienophiles. We predicted
that diene 2 would have a better
reactivity profile, but we were unable to successfully achieve a useful
synthesis.
Consequently, we turned our focus
to the synthesis of cyclic dienes adjacent to cobalt-complexed alkynes. We predicted that these would be easier to
prepare and more reactive in Diels-Alder reactions. We synthesized diene 5 in 2 steps from 5-bromofuraldehyde (3), and we could easily cobalt-complex the alkyne in 5 to yield diene 7.
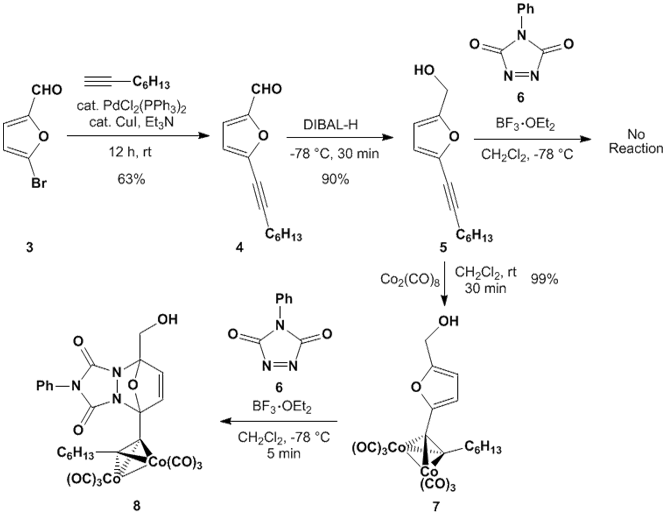
Diels-Alder reactions of dienes 5 and 7 with the highly reactive dienophile PTAD (6) supported our hypothesis that cobalt-alkyne complexes adjacent
to dienes in the Diels-Alder reaction will enhance their reactivity. Uncomplexed diene 5 is unreactive with PTAD at -78 °C in the presence of boron
trifluoride. However, cobalt-complexed
diene 7 reacts completely in 5
minutes under identical conditions.
Isolation of cycloadduct 8
proved problematic under the reaction conditions; we believe that this cyclic
ether undergoes undesired fragmentation or rearrangement process in the
presence of boron trifluoride.
Nevertheless, these results clearly demonstrate that a cobalt-alkyne
adjacent to a diene has a dramatic rate enhancement effect in the Diels-Alder
reaction. Studies are currently underway
to investigate similar reactions of substituted cyclohexadienes that should
yield Diels-Alder products that are more easily isolable.
The second project that has been previously
described in PRF annual reports focuses on a tandem intramolecular
Diels-Alder/Pauson-Khand strategy for the synthesis of the tetracyclic steroid
skeleton. Unfortunately, we have been
unable to investigate the key Diels-Alder/Pauson-Khand steps because of the
failure of one crucial coupling reaction (9
+ 10 → 11).
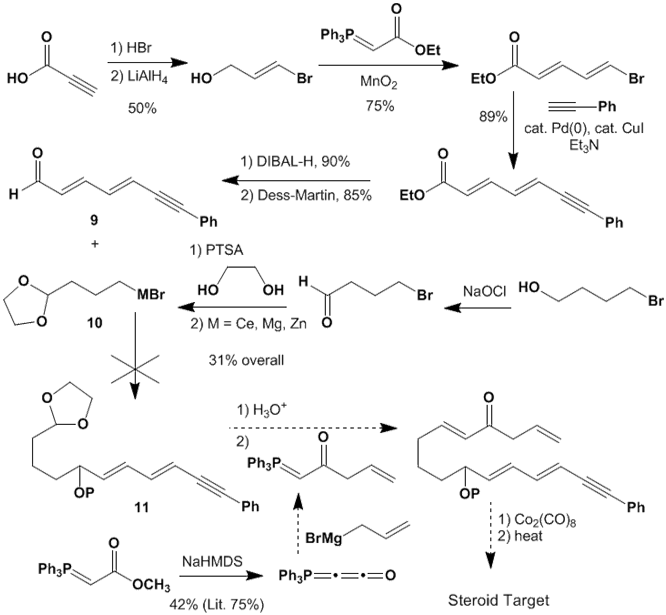
We explored
numerous options for the construction of this critical carbon-carbon bond. In addition to the route shown here, we
initially attempted to couple the allylic acetate analog of aldehyde 9 with Grignard 10 in the presence of a copper catalyst. The second generation approach pictured above
focused on the direct addition of organometallic 10 to the aldehyde of 9. However, all of our attempts proved
fruitless. No improvement was observed
when varying the nucleophiles from a Grignard reagent, to an organozinc
compound, or to an organocerium reagent.
We plan to address this problem by synthesizing the Weinreb amide analog
of aldehyde 9 in hopes that it will
react as desired with organometallic reagents 10.
We have recently initiated an investigation into the
behavior of cationic Diels-Alder dienophiles stabilized by adjacent cobalt-complexed
alkynes. Gassman demonstrated that
alkenes conjugated to oxonium ions serve as excellent dienophiles, and we hoped
to achieve similar results with alkenes conjugated to cobalt-complexed alkyne-stabilized
carbocations (i.e., 12).
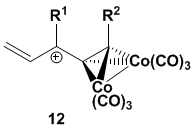
We planned to generate the
requisite carbocation by the ionization of a cyclic
ether which could reform the ether after participating in the Diels-Alder
reaction. After a thorough literature
search for known molecules with the desired enyne
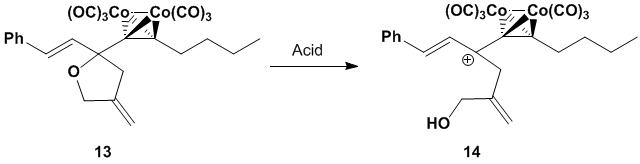
ether functionality we desired, we identified cyclic ether 13 as our dienophile precursor.
We predicted that 13 would
react with Lewis and/or protic acids to provide
cationic dienophile 14.
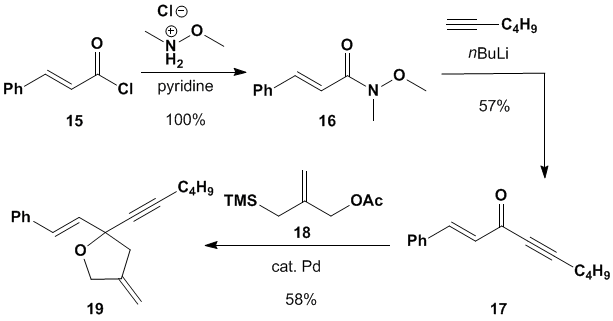
Following a synthetic procedure
from Trost's lab, we converted cinnamoyl
chloride (15) into dienyne 19 in
three steps.
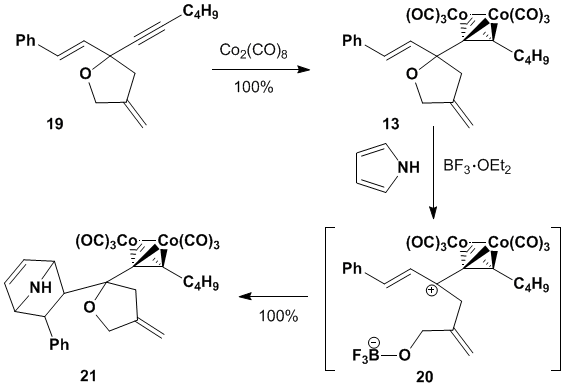
Alkyne 19 was easily converted into cobalt-alkyne complex 13 upon exposure to dicobalt
octacarbonyl.
Treatment of 13 with boron
trifluoride generates cationic dienophile 20
that combines with pyrrole in a Diels-Alder reaction
to provide cycloadduct 21. We are currently investigating the
stereochemical outcome of this reaction.
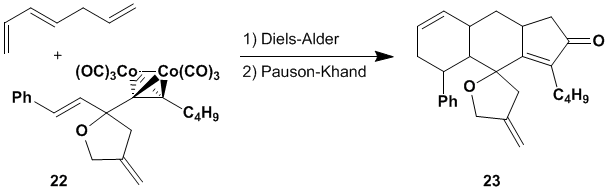
Excited by this result, investigations
are underway with numerous other dienes as well as attempts to make a variety
of similar dienophiles. One interesting
application, shown below, involves a tandem combination of the Diels-Alder and
Pauson-Khand reactions. For example, reaction
of 1,3,6-heptatriene with cobalt-complexed alkyne 22 should yield tetracycle 23 after a tandem
Diels-Alder/Pauson-Khand process. We
hope to explore this reaction in the near future.
Back to top











