

45063-G9
The Interfacial Structure of Water on a Hydrophobic Surface: A Mechanistic Study
This research aims to understand the interfacial molecular structure and water transport near the surfaces of varied hydrophobicity by complementary force-sensing and microscopic approaches.
In the second and final year of the grant, we have completed the characterization and mechanistic study of complex macromolecular self-assembly and water transport of polymeric aqueous suspensions at the water-solid interfaces upon evaporation. We have also examined the AC-electric field enhanced wetting process of electrolyte and polyelectrolyte aqueous solutions at microelectrod-embedded surfaces. Our key accomplishments are highlighted below.
 Over the two-year grant period, we have completed the study
of the interfacial transport and assembly at the water-solid interfaces. As we
continue the study of evaporative self-assembly of
periodic DNA multiple-rings from the first year, we extend the research from
single nonvolatile DNA in aqueous suspensions to DNA-silica particle binary
systems. Specifically, we examine the effects of colloidal particle size and surface
charge as well as the concentrations of both DNA and colloidal particles on the
microstructure generated from evaporating the aqueous droplets of binary
components. We have demonstrated that the composition
of the binary system plays a critical role on the periodic multiple-ring
formation in the following aspects: 1) Suspensions with high DNA concentrations
and low colloidal concentrations favor the formation of multiple-ring pattern,
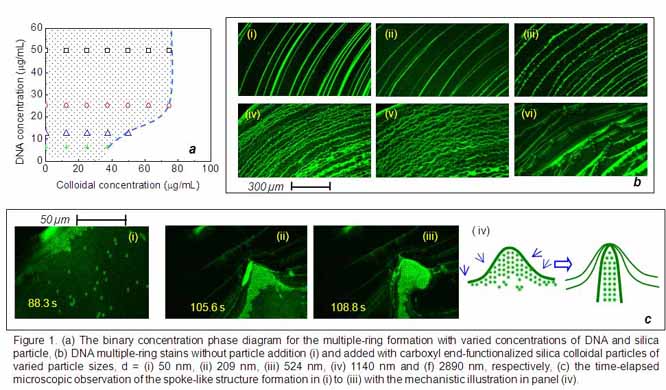
as indicated by the phase diagram shown in Fig. 1a, 2) the size of colloidal
particles added into DNA aqueous droplets can significantly disrupt smooth
multiple rings to form rippled rings and curtain-like periodic patterns with a
curious spoke-like structure as the size of colloidal particles increases, as
exhibited in Fig. 1b-(i)-(vi); and 3) the enhancement of DNA-colloid
interaction by opposite charged colloidal particles result in considerably high
irregularity of DNA stain ring spacing. We also have examined the disruption of
multi-ring morphology under varied conditions and attribute it to local
hydrodynamics governed by colloid aggregation and sedimentation: Small
particles tend to be embedded into the DNA molecular network while large
particles segregat from the primary DNA rings and result in a spoke-like
structure, whose evolution was investigated via real-time microscopic
observation as shown in Fig. 1c-(i)-(iv). Two journal papers
based on this work was recently published in Phys. Rev. Lett (2008) and
Langmuir (2008).
Over the two-year grant period, we have completed the study
of the interfacial transport and assembly at the water-solid interfaces. As we
continue the study of evaporative self-assembly of
periodic DNA multiple-rings from the first year, we extend the research from
single nonvolatile DNA in aqueous suspensions to DNA-silica particle binary
systems. Specifically, we examine the effects of colloidal particle size and surface
charge as well as the concentrations of both DNA and colloidal particles on the
microstructure generated from evaporating the aqueous droplets of binary
components. We have demonstrated that the composition
of the binary system plays a critical role on the periodic multiple-ring
formation in the following aspects: 1) Suspensions with high DNA concentrations
and low colloidal concentrations favor the formation of multiple-ring pattern,
as indicated by the phase diagram shown in Fig. 1a, 2) the size of colloidal
particles added into DNA aqueous droplets can significantly disrupt smooth
multiple rings to form rippled rings and curtain-like periodic patterns with a
curious spoke-like structure as the size of colloidal particles increases, as
exhibited in Fig. 1b-(i)-(vi); and 3) the enhancement of DNA-colloid
interaction by opposite charged colloidal particles result in considerably high
irregularity of DNA stain ring spacing. We also have examined the disruption of
multi-ring morphology under varied conditions and attribute it to local
hydrodynamics governed by colloid aggregation and sedimentation: Small
particles tend to be embedded into the DNA molecular network while large
particles segregat from the primary DNA rings and result in a spoke-like
structure, whose evolution was investigated via real-time microscopic
observation as shown in Fig. 1c-(i)-(iv). Two journal papers
based on this work was recently published in Phys. Rev. Lett (2008) and
Langmuir (2008).
Related to the work of evaporative macromolecular self-assembly at water-solid interfaces, we also have developed a novel method to effectively stretch DNAs at water-solid interfaces via combined electrospraying and micro-droplet evaporation. By employing the electrospraying technique, we have generated a large number of micron and submicron-sized aqueous droplets on a solid substrate. Subsequently, we have observed the elongation of DNA molecules with high stretch ratio inside and outside DNA aqueous droplets by the combination of three hydrodynamically-facilitated modes: (i) uncoiled DNAs in the tail of rolling micro-droplets immediately after electrospraying, (ii) spoke-like DNAs adjacent to moving contact lines at water-solid interfaces, and (iii) molecular combing of DNAs inside evaporating micro-droplets. A manuscript is currently in preparation.
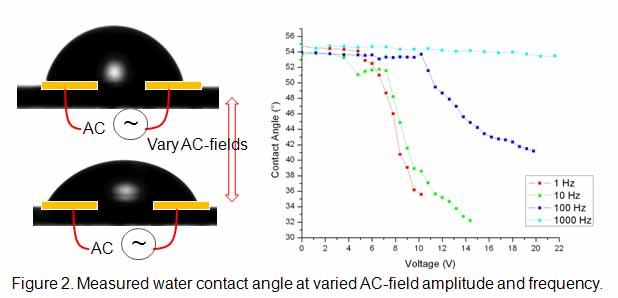
In parallel, we have examined the AC-field dependent electrowetting of aqueous droplet at partially hydrophobic surfaces embedded with gold microelectrode arrays. As shown in Fig. 2, the water contact angle of electrolyte aqueous solutions placed between two microelectrodes are observed to decrease as increasing AC-field amplitude after a threshold amplitude and frequency is exceeded. The scaling behavior with varied droplet size and medium conductivity of electrolyte aqueous solutions are explored to quantify the contribution of the dynamic double-layer effect on AC-electrowetting: The critical AC-frequency for enhanced electrowetting is found to scale with D/(la), where D is ionic diffusivity, l is the Debye length and a is the radius of the sessile droplets. In addition, the ejection of nano-droplets from parent sessile droplets is observed due to the Reyleigh instability by the charge accumulation at the contact line. A manuscript is recently submitted to Phys. Rev. Lett.
With the success of this funded research on interfacial self-assembly and wetting process of aqueous suspensions, we have achieved an much improved understanding, at a microscopic level, of the effects of solute components, interfacial flow rates and applied AC-electric fields on the interfacial macromolecular assembly at water-solid interfaces. The study of AC-electrokinetic effects on interfacial wetting has opened a new research area in the PI's laboratory to examine the AC-polarization of polyelectrolyte molecules in aqueous medium, where ionic re-distribution near the charged polymer chain by applied AC-fields is expected to affect the interfacial conformational transition and assembly. Some data obtained from the interfacial AC-electrowetting study under this ACS PRF type-G grant have been used as preliminary results for the grant applications submitted to federal agencies including the NSF CAREER award. This grant is leveraged by other research grants the PI has to support participating graduate students.