44936-GB5
Nanotribology Studies of Petroleum Derivatives for Nanotechnology
During our research into the adsorption of alcohols atop self assembled monolayers (SAMs) and metals, we noticed some problems with the commercially available quartz crystal microbalances (QCMs). The SiO2 films were quite porous, and QCM measurements of viscosity (using various QCM substrates) did not match the known bulk viscosity of the fluids. This caused problems for our study of the alcohols, but opened up a new area of research for us: the study of the effect of nano-bubbles and surface roughness on QCM fluid measurements. This became the masters thesis of my graduate student, Jon Jones. He finished his thesis in May of 2008, and is now happily employed as an engineer in Asheville, NC. I summarize the purpose and results to date of this study below. Jon and I are currently preparing a paper on this subject for either the Journal of Colloid and Interface Science or perhaps the Journal of Physics: Condensed Matter.
Recently there has been much interest in the presence of so-called nano-bubbles on solid surfaces submerged in liquids. Prior work in this area has mainly consisted of scanned probe microscopy studies [Tyrrell & Attard, PRL 87 (2001); Simonsen et al., J. Coll. Inter. Sci. 273 (2004)]. Our study shows that macro- and nano-scale bubbles can be detected by Quartz Crystal Microbalances (QCM) submerged in liquids. QCMs are high quality factor oscillators that generate a shear wave when submerged in a fluid. The damping of the oscillation by the fluid causes the resonance frequency of the QCM to shift, and a viscosity can be calculated from the frequency shift. Since the shear wave generated is so short, this is an interfacial viscosity as opposed to a bulk viscosity. Due to increasing use of QCMs in solid/liquid studies and because of their increasing popularity as viscometers in both industry and academia, it is important to consider the possibility that nano-bubbles may be forming on the surface causing shifts in measurements. The effects of cleaning and surface preparation techniques must also be considered when using QCMs as viscometers.
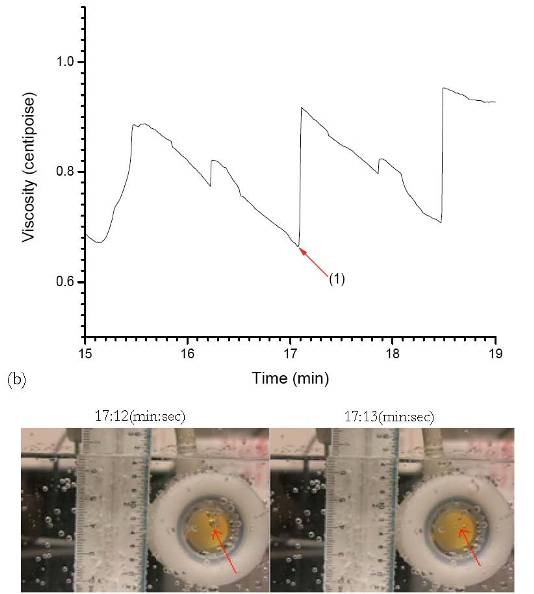
In this study we have submerged QCMs with both hydrophilic (gold) and hydrophobic (octadecanethiol) surfaces in water distilled to varying degrees, including seltzer water and vacuum distilled DI water. The seltzer water has visible macro-scale bubbles, and so we were able to correlate video acquired of the QCM submerged in the seltzer water with the viscosity data to directly link the presence or absence of a bubble to a change in the measured viscosity. As bubbles formed on the hydrophobic QCM electrode, the measured interfacial viscosity decreased because the viscosity of air is orders of magnitude smaller than the viscosity of liquid water. In the figure taken from our video shown below, the seltzer bubble indicated by the red arrow popped, resulting in an upward jump in the interfacial viscosity.
We were not able to image, visually
or otherwise, the presence of smaller bubbles on the QCM electrode, but the
nano-bubbles' presence can still be shown via the reduced measured interfacial
viscosity of the liquid. In order to
examine the effects of nano-scale bubbles, we vacuum distilled de-ionized
water, and then measured its viscosity with both a hydrophilic and hydrophobic
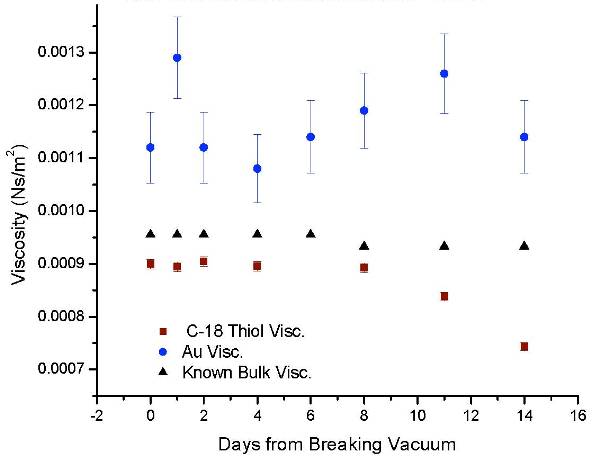
QCM for 2 weeks after breaking vacuum (shown in figure below).
Like the macro-bubbles, the nano-bubbles
present in the distilled water caused a reduced measured interfacial
viscosity. This viscosity dropped as
time increased due to the re-saturation of air into the distilled water. Interestingly,
the interfacial viscosity measured by the hydrophilic crystal was always higher
than the known bulk viscosity water at the given temperature. The cause of this
increased viscosity is unknown.
Possibilities include an increased water density due to the very hydrophilic
surface or small surface roughness effects.
We are currently finishing up studies of small surface roughness effects
caused by QCM preparation techniques.
This work combines scanning electron microscopy, atomic force
microscopy, and QCM studies. Upon
completion of this work, we will include our results with Jon's masters thesis
results and publish our findings, as stated above.
The monies from Petroleum Research
Fund allowed me to employ Charles Little (Cully), an undergraduate physics
student, and Jon Jones, a physics graduate student, over the last two
years. Cully graduated with his B.S. in
physics in May of 2008. Originally Cully
planned to get a job after his undergraduate work, but his experience as an
undergraduate researcher has inspired Cully to go to graduate school in
physics. He is currently applying to
graduate schools and continuing to work in my lab in his free time. In addition to helping my students advance
their careers, Petroleum Research Fund monies have enabled me to purchase
valuable equipment to further the research summarized above and publish in peer
reviewed journals, which has been invaluable to my career as a scientist.