

45642-AC4
Improving Terephthalic Acid Synthesis in High-Temperature Water
Introduction
Terephthalic acid is a commodity chemical, and its main use is in the production of polyethylene terephthalate, a polymer used to make synthetic fibers and beverage containers. Terephthalic acid is produced commercially in acetic acid, and then purified by hydrogenation in high-temperature water. If water were used as the medium for the synthesis step, a simpler process may be possible because there would be no need to change solvents for the two steps. Moreover, water is a byproduct from the oxidation so the current process includes a tall distillation column for separating acetic acid, so it can be recycled, and water. If water were the reaction medium for terephthalic acid synthesis, the need for this column and its operating costs would be eliminated. The cost of this tower is significant both because of its size and its internals requiring titanium to withstand the corrosive conditions. Finally, the bromide catalysts used react with acetic acid to form small amounts of methyl bromide. This pollutant must be removed from the gaseous effluent stream. If water were the solvent, no methyl bromide would form so the process would be less polluting. There are both environmental and economic payoffs if terephthalic acid synthesis were to be practiced in water commercially.
Background
Previous work in our lab has demonstrated that high-temperature liquid water (HTW) at 300 °C is a very promising medium. High terephthalic acid yields (80 – 90%) and very high selectivities (essentially 100%) are available. Though the technical feasibility of a HTW process for terephthalic acid synthesis has been demonstrated, the reaction must be improved and further refined if it is going to displace the current acetic acid based process, which has benefited from years of continuous refinement and optimization.
One barrier that accompanies the HTW process at present is the low p-xylene concentrations (about 0.02 mol/L) that have been used experimentally. Low p-xylene concentrations mean low production of terephthalic acid per unit reactor volume. Thus, there is a need to explore synthesis at higher concentrations of p-xylene to get greater productivity. Work to date on this project has focused on this objective of increasing productivity of terephthalic acid synthesis in HTW by running at high p-xylene concentrations.
To work at higher reactant concentrations, though, involves running the reaction as a two-phase aqueous suspension since higher concentrations exceed the solubility of p-xylene in HTW. It is not known whether this two-phase processing scheme will be successful.
Results
We have conducted several sets of stirred, batch oxidation experiments at 300 °C with p-xylene concentrations higher than previously explored. Experiments were done with p-xylene loadings ten times and 20 times higher than those used in previous research. The reactor was initially loaded with p-xylene, water, and catalysts. An important feature of our experiments is that the oxygen gas was added to the pre-heated and pressurized reactor contents. We tested both incremental addition in small bursts and continuous oxygen addition. Table 1 summarizes the experimental conditions and the results. The product yields and selectivities in the table are those determined from analysis of the material remaining in the reactor at the end of the run. The results show that high yields and high selectivities (> 90%) to terephthalic acid are available only via incremental addition of oxygen to the reactor system. Continuous addition of oxygen (runs 4 – 7) invariably led to low yields and low selectivities. Nevertheless, we have been successful in identifying synthesis conditions that form high yields of terephthalic acid in HTW even at 10- and 20-fold higher p-xylene concentrations than used previously.
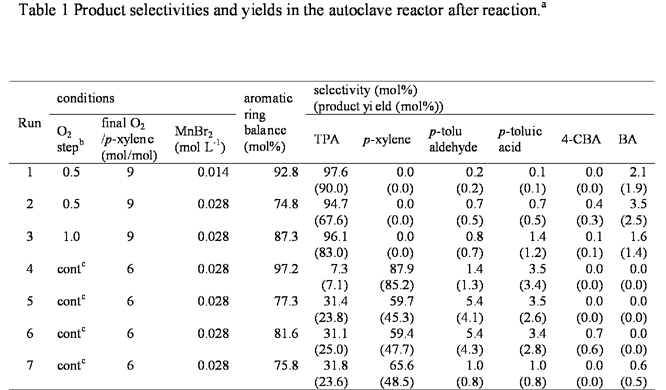
a Reaction condition; p-xylene 0.2 mol L-1.
b The molar ratio of O2 added to p-xylene initially loaded. The stoichiometric amount needed to convert p-xylene to TPA is 3.
c cont = continuous addition of O2