

44770-AC3
Heterobimetallic Bismuth-Transition Metal Carboxylates as Catalysts and Catalytic Precursors
Work over past year has been focused on two types of heterometallic bismuth - transition metal compounds: Bi-Rh catalysts and Bi-Pd catalytic precursors.
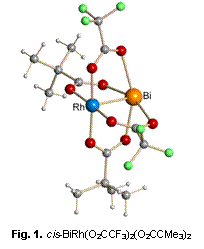
We have continued our research on heterometallic bismuth(II)-rhodium(II) carboxylates, BiRh(O2CR)4. These remarkable molecules have been shown to maintain a fully-ordered paddlewheel structure with a single Bi–Rh bonding interaction (Figure 1). The air-stable complexes retain their heterometallic structure in solution and exhibit an avid one-end Lewis acidity at the rhodium site only. In addition to the solvent-free preparative procedure reported earlier, the solution method exemplified by the equation (1) has been developed for the synthesis of heterometallic bismuth-rhodium carboxylates.
2Bi + 4Bi(O2CCF3)3 + 3Rh2(O2CCF3)4 → 6BiRh(O2CCF3)4 (1)
 This technique has several
major advantages over the solid-state procedure. First of all, the process is
easy to scale-up to gram quantities, which is one of the requirements for
practical catalyst. Second, no additional steps are needed to get an unstable
and hard-to-handle intermediate Bi2(O2CCF3)4.
Importantly, by cutting the preparation sequence to only one step the reaction
time is reduced, while the overall yield is improved. Finally, the volatility
of starting materials and products is not important for the above solution
process. That allows to use the above reaction conditions for other bridging
ligands and, possibly, for other metal combinations. The latter opens a broad
venue of opportunities, for instance, an access to chiral mixed-metal
catalysts.
This technique has several
major advantages over the solid-state procedure. First of all, the process is
easy to scale-up to gram quantities, which is one of the requirements for
practical catalyst. Second, no additional steps are needed to get an unstable
and hard-to-handle intermediate Bi2(O2CCF3)4.
Importantly, by cutting the preparation sequence to only one step the reaction
time is reduced, while the overall yield is improved. Finally, the volatility
of starting materials and products is not important for the above solution
process. That allows to use the above reaction conditions for other bridging
ligands and, possibly, for other metal combinations. The latter opens a broad
venue of opportunities, for instance, an access to chiral mixed-metal
catalysts.
The catalytic activity of heterometallic Bi-Rh carboxylates in the reactions of diazo transformations has been tested and compared with that of homometallic dirhodium counterparts. It has been found that heterometallic compounds exhibit behavior very similar to the dirhodium analogs in reactions of cyclopropanation of olefins or arenes and in aliphatic or aryl C–H insertions when using donor-acceptor carbenoids. This result is important because it sheds the light on the role of the second rhodium atom in paddlewheel dimetal unit. It confirms the idea that the dirhodium catalyst uses only one of its two coordination sites at a time for carbene binding, while the second atom acts as an anchor for the bridging carboxylate ligands and as an electron pool in the carbenoid intermediate formation.
 Bismuth has
long been known to promote the activity of palladium catalysts impregnated onto
the solid support. We have been working on the synthesis of heterometallic
compounds that are capable of providing a direct interaction between the
palladium atoms and support surface functional groups while allow to achieve a
high level of catalyst dispersion upon thermal treatment.
Bismuth has
long been known to promote the activity of palladium catalysts impregnated onto
the solid support. We have been working on the synthesis of heterometallic
compounds that are capable of providing a direct interaction between the
palladium atoms and support surface functional groups while allow to achieve a
high level of catalyst dispersion upon thermal treatment.
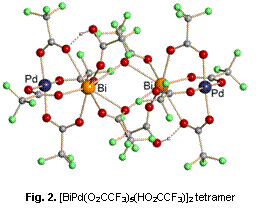
By reacting palladium(II) carboxylates with bismuth(III) carboxylates in the solid state, we have isolated a new family of mixed-metal complexes having general formula BiPd(O2CR)5. The structure of the trifluoroacetate complex as an adduct with trifluoroacetic acid has been determined and revealed a heterometallic tetramer [BiPd(O2CCF3)5∙(HO2CCF3)]2 (Figure 2). The palladium atoms in this molecule maintain an open coordination site that is available for interaction with functional groups on support surface. The complex is air-stable, highly volatile, and is soluble in a variety of common solvents. In solutions of coordinating solvents, the tetrameric molecule transforms to the dinuclear BiPd(O2CCF3)5×3L species. The TGA/DTA analysis reveals clean decomposition completed at ca. 350 oC while showing no apparent loss of material to sublimation. The analysis of inert atmosphere decomposition products indicates the presence of metallic palladium and bismuth(III) oxofluoride phases.
The catalytic performance of the sample prepared by grafting of the heterometallic Bi-Pd precursor onto functionalized carbon support has been tested in the model reaction of D-glucose/D-gluconic acid liquid phase oxidation. The catalyst obtained from the heteronuclear Bi-Pd complex has demonstrated excellent catalytic activity when compared to the reference samples prepared from the mixture of homometallic carboxylates.