46716-B3
Synthesis, Coordination Chemistry, and Catalytic Studies of a Library of Water-Soluble Polydentate Phosphines Derived from 1,3,5-Triaza-7-phosphaadamantane (PTA)
The greening of industry has caused recent research to center around the
development of water-soluble phosphines and their transition metal
catalysts. One ligand that has been
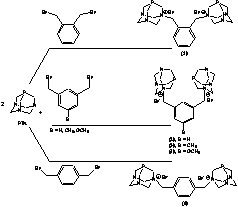
extensively studied is Scheme 1. Synthesis of the Bis-PTA xylenes. Compounds 3 and 4 were additionally
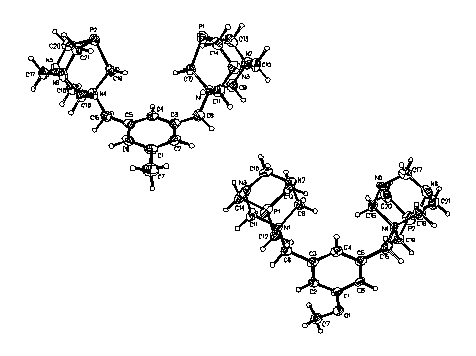
characterized via single crystal X-ray diffraction. The
geometry about the P atoms of both compounds was compressed pyramidal, as
evidenced by their small C-P-C bond angles.
The average tertiary nitrogen, N-C bond distance within 3 (1.460(6) Å) and 4 (1.458(8) Å) was quite comparable, and slightly shorter than
those involving the quaternary nitrogen (1.530(5) and 1.533(7) Å). The PTA moieties of 3 and 4 were orthogonal
to the plane of the phenyl rings as evidenced by the average C-N-C-C(ring)
torsions angles of 91.5o (5) and 89.9o (6) respectively.
Figure 1. Thermal ellipsoid representations of 3 and 4. The bromide anions and solvate molecules have been omitted for
clarity. The primary purpose of this study
was to prepare bis-phosphines that could possibly function as water-soluble
ligands. Therefore, the
solubility of compounds 1-5 was studied in aqueous media. The substitution pattern of the aromatic ring had a
pronounced effect on the water-solubility of the phosphines with the ortho
compound 1 being very water soluble
(2000 mg/mL) and the para analog (5)
far less (12.5 mg/mL). The meta phosphine 2
and its tolyl analog (3) were
found to have water-solubilities (810 mg/mL) that were triple that of PTA (235
mg/mL). The difference in water
solubility between the ortho (1),
meta (2), and para (5) substituted compounds is quite
remarkable. It is hypothesized that this
is a consequence of the varied lattice enthalpies of the related systems. The anisolyl compound (4) was determined to be much less soluble (121 mg/mL) than the
other meta derivatives, thus indicating that, in addition to the substitution
pattern of the ring, the substituents on the ring greatly impact the
water-solubility of the compounds. It is assumed that the low solubility of 4 was a consequence of the OCH3
moiety hydrogen bonding with water to form a polymeric network. In addition to adding five new phosphines to the library of
water-soluble ligands, this study also had great impact on my research
program. First, it allowed four
undergraduates to experience the thrill of preparing and analyzing molecules
that had never been synthesized. Two of
the students are continuing to work with me this year, and a third went to
graduate school to earn her Ph.D. in synthetic chemistry. Second, this project was important in that it
created a new collaboration between my lab and that of Dr. Paola Bergamini of
the