
Back to Table of Contents
45830-GB7
Probing Through-Space Charge Migration in Segmented Conducting Polymers
Jocelyn M. Nadeau, Marist College
The discovery of conducting
polymers in the 1970s has led to countless important technological applications
including organic light-emitting diodes, chemical sensors, and corrosion
inhibitors. Using segmented conducting
polymers composed of electroactive segments connected by insulating segments, our
goal is to study how the relative orientation of the electroactive segments
modulates the electrical and optical properties of the polymers. Toward this aim, undergraduate coworkers and I have established a successful route to C-shaped electroactive
monomer 1 and are currently working on the synthesis of electroactive monomer
2 (Figure 1).
Electropolymerization of these monomers is expected to lead to segmented
conducting polymers with well-defined structures that will be used to study
through-space charge migration.
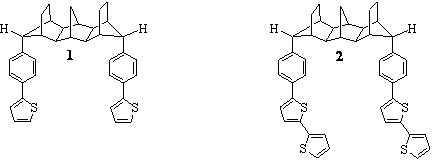
Figure
1. C-Shaped electroactive monomers, 1 and 2.
Our first, and perhaps most important, task was to
synthesize dibromide 3, where C-shaped 3 is the basic building
block from which a family of desired C-shaped electroactive monomers will be
obtained (Figure 2; left). Dibromide 3
was produced as a mixture of diastereomers, where the major isomer
comprising 71% of the mixture is C-shaped (NMR integration).

Figure 2. (left)
Structure of key synthetic intermediate 3. (right) ORTEP drawing of
C-shaped 3 from preliminary X-ray diffraction data (crystal system:
triclinic; space group: P-1).
Evidence supporting the C-shaped topology of the
major diastereomer of 3 comes from preliminary X-ray diffraction data
(Figure 2; right). A molecule of CDCl3,
the crystallization solvent, can be seen occupying the cleft between the
aromatic rings, which is a promising result because we anticipate that poly(1)
and poly(2) might show promise in sensing applications. Although some progress has been made toward isolating
pure C-shaped 3 using column chromatography and recrystallization, studies
are currently underway to determine conditions for separating the diastereomers
by preparative HPLC. Pd-catalyzed Suzuki
coupling of 3 with 2-thiopheneboronic acid gave C-shaped monomer 1
in 60% yield. Preliminary
electrochemical studies showed that the redox potential of 1 is too high
to allow for electropolymerization within the solvent window, and our solvent
selection is limited due to poor monomer solubility. Monomer 2 is expected to have a lower
redox potential due to the additional thiophene rings, which should favor
electropolymerization at a lower potential.
Although our current focus is on the synthesis of 2, we will also explore alternatives for obtaining
poly(1) either chemically or electrochemically.
We
became interested in another family of electroactive monomers, 4 and 5, that we have synthesized and characterized (Figure 3). These axially
chiral monomers are intriguing because their electropolymerization leads to chiral
segmented conducting polymers wherein the electroactive segments are constrained
to be perpendicular to each other along the polymer backbone.
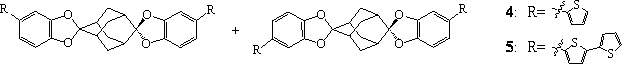
Figure 3. Structures of 4 and 5.
Electropolymerization of 4
and 5 using cyclic voltammetry led
to poly(4) and poly(5), respectively, where both polymers exhibited
robust growth with each successive scan.
Spectroelectrochemistry studies demonstrated that both polymers are
electrochromic. We will continue to
study charge migration in poly(4)
and poly(5) to assess the effect
this new polymer topology has on their electrical and optical properties.
This
funding has had a huge impact on my research career and on the blossoming
careers of my undergraduate coworkers.
Although I received start-up support from Marist, this money was quickly
exhausted on glassware, chemicals, and supplies for synthesis. In addition to summer stipends, including a
SUMR supplement, the PRF funding has allowed us to initiate the
electrochemistry thrust of the research.
I purchased the electrochemistry equipment and associated accessories,
including several costly precious metal electrodes, using a combination of PRF
and matching funds from the College. The
four undergraduates who have worked on the project gained valuable experience by
their direct participation at all levels of the research, including critical analysis
of the primary spectroscopic and electrochemical data. They have not only been enriched by the lab
experience, but they have all had opportunities to present their research at off-campus
venues. Most notably, my three summer 2008
researchers presented posters at the 236th National ACS Meeting in Philadelphia, PA. All four undergraduates intend to pursue a
PhD in chemistry, where one is already at the U. of Cincinnati
and two are applying fall 2008.
Back to top






