

46294-G3
Study the State of Counter-ions in Polyoxometalate Solutions
<> This project is aiming for understand the role of small counter-ions in the solutions of hydrophilic polyoxometalate (POM) macroions, which are widely used as catalysts in petroleum industry and as novel functional nanomaterials. We have made the following progresses in the past fiscal year:
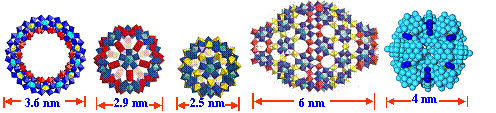
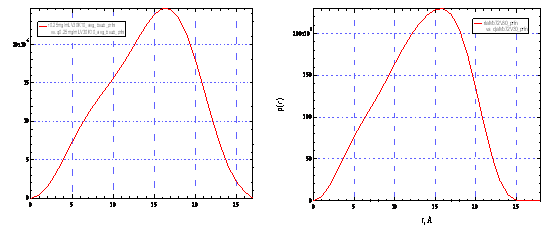
1. Radial distribution of small cations around POM
macroanions
<>
We have successfully obtained beamtime as a general user at Advanced Photo Source, Argonne National Laboratory to perform synchrotron X-ray scattering experiments. For the first time, we obtained the radial distribution of small metal cations around POM macroions (2.5-nm-radius, spherical “Keplerate” POM {Mo72V30}, with -31 charges). The major results are:
(1) (2) (3) The appearance of the counter-ion association around POM
macroions is coincident with the self-assembly of the POM macroions into
“blackberry” structures in solution. This is strong evidence that the
blackberry formation, a type of quite universal self-assembled structures by
hydrophilic macroions, is driven by the counter-ion-mediated attractions. The
work will be submitted to Phys. Rev. Lett.
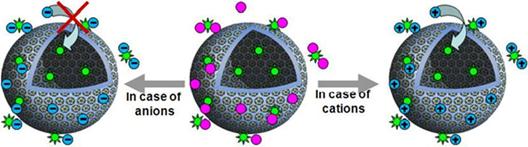
2. Counter-ion transport over the “blackberry” membrane
The blackberry structure itself is a hollow, porous
sphere because the macroions are not touching with each other on the blackberry
surface. Consequently, the blackberry surface can be treated as a special
membrane. By using fluorescence and light scattering techniques, we found that
the blackberry “membrane” is very unique because it allows the slow transport
of small cations (such as Ca2+ and Mg2+) from the bulk
solution into the space inside the blackberries. This is completely different
from bilayer cell membranes which are not permeable to cations and special
carriers are needed. Also, we find that the water encapsulated into the
blackberry shells has a higher viscosity than that in bulk solution, possibly
due to the different hydrogen bondings. The results are published in J. Am.
Chem. Soc. 2008, 130, 1548.
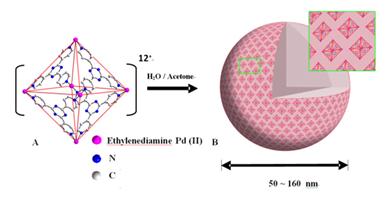
<> 3. Self-assembly of metal-organic macrocations
Additional to the polyoxometalate macroanionic systems
we have extensively studied, we want to explore whether the unique,
counter-ion-mediated self-assembly of macroions into blackberry-type structures
is universal for other types of systems. We have studied the solution behavior
of (Wako) Pd6L4 {Pd = Pd(ethylenediamine), L =
2,4,6-tris(4-pyridyl)-triazine} metal-organic nanocages in the mixed solvents
of water and acetone. Interestingly, blackberry formation was observed when
certain acetone was introduced. The blackberry size increases with increasing
acetone content, same as that in POM macroanionic solutions, indicating a
charge-regulated self-assembly process. The results confirm that the blackberry
formation is universal. The unique structure of the nanocages enables us to
expand our study of macroions by considering of loading signal-sensitive
materials. The results are published in J. Am. Chem. Soc. 2008,
130, 4226.
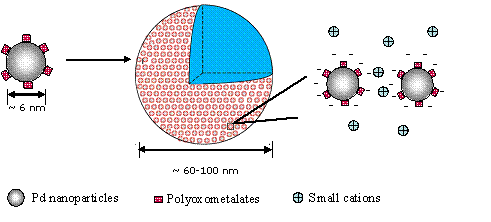
<> In this work, we demonstrate that the self-assembly of
macroions into blackberry-type structures can be achieved by hydrophilic
nanoparticles. The hydrophobic Pd nanoparticles become hydrophilic when coated
with Dawson-type V-substituted POM K9[H4PVIVW17O62]
(HPVIV) clusters. This work is published in Langmuir 2008,
24, 5277.
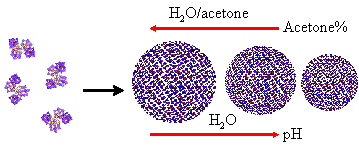
{P4Y8W43} is a type of
large polyoxotungstate. We find that it is unique because they demonstrate the
properties of both “strong electrolyte” and “weak electrolyte” types of POMs in
solution. Consequently, they can form blackberries and the blackberry size can
be adjusted by either changing pH or changing solvent content. Thus it can be
used as a valuable system to directly the effects of the two processes. The
work is published in Langmuir 2008, 24, 9308.
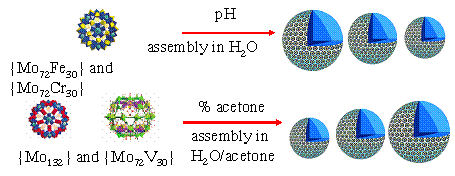
Three Keplerate types of structurally similar giant POM
clusters, {Mo72Fe30}, {Mo72V30} and
{Mo72Cr30}, are compared for their solution behavior. {Mo72Fe30}
and {Mo72Cr30} are similar in solution, both showing
weak-acid features due to the partial deprotonation of their water ligands. {Mo72V30}
is also a weak acid, but more or less behave like a weak acid salt due to the
large amount of inherent charges inside its skeleton. The work helps to
summarize the self-assembly of such large, hollow, spherical POM macroions in
solution. The manuscript is submitted.
By chemically attaching organic ligands to the large
POM clusters, novel inorganic-organic hybrids can be synthesized. Such hybrids
could show amphiphilic properties due to the hydrophilic POM clusters and the
hydrophobic alkyl chains. For the first time, we confirm such behavior by
observing the formation of bilayer vesicles formed by [n-Bu4N]3[MnMo6O18{(OCH2)3CNHCO-(CH2)14CH3}2]
(Mn-Anderson-C16) hybrids in water/acetonitrile mixed solvents. The
manuscript is submitted.
<>
 <>4. Self-assembly of POM-coated Pd
nanoparticles
<>4. Self-assembly of POM-coated Pd
nanoparticles
5. Self-assembly of Yttrium-containing polyoxometalate (K15Na6(H3O)9[(PY2W10O38)4(W3O14)]×9H2O, or {P4Y8W43})
macroanions in aqueous solution
6. Comparison among three types of POM giant “weak acids”
7. POM-organic hybrid materials
