44139-GB3
Doubly Bridged Metallocenes with Two Distinct Interannular Linkers: Synthesis and Regioselectivity for alpha-Olefin Polymerization
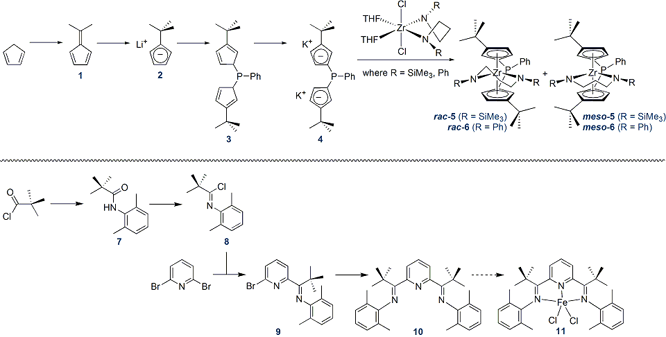
During the past year, my research group has pursued the synthesis of two sterically hindered ligands and their corresponding metal complexes. These targets are: (1) singly bridged zirconocenes with one bulky phenyl-phosphine interannular linker (described in year 1 report), and (2) a novel pyridine bis(imine) ligand with t-butyl substitution on both imino carbon atoms, and attempted metallation with iron(II)chloride (mentioned with minimal detail in year 1 report). With the success of these projects during the year 2 grant period, the preparation of doubly bridged zirconocenes with two distinct interannular linkers was pursued less intensely to allow the former two projects to develop to the stage necessary for publication. However, the singly linked ligand for project 1 is a synthetic precursor to a doubly bridged zirconocene with two distinct interannular linkers, and therefore project 1 provides some valuable insight for our long term synthetic goals.
During year
2, the chemistry for project 1 was developed sufficiently for publication. The
synthesis of neutral ligand, 3, and dipotassio salt, 4, was
carried out reproducibly and both were characterized by 1H, 13C{1H},
and 31P{1H} NMR spectroscopy. Syntheses of rac/meso-{PhP(3-t-Bu-C5H3)2}Zr{Me3SiN(CH2)3NSiMe3}
(rac-5/meso-5, in a 2 to 1 ratio) and rac/meso-{PhP(3-t-Bu-C5H3)2}Zr{PhN(CH2)3NPh}
(rac-6/meso-6, in a 1 to 3 ratio) was achieved by metallation of
4 with Zr{RN(CH2)3NR}Cl2(THF)2
(where R = SiMe3 or Ph, respectively), using the chelate-controlled
metallation method described previously by
Professor Richard F.
Jordan (University of Chicago).
Again, multinuclear NMR spectroscopy
was used for characterization; both rac-5 and rac-6
were further analyzed crystallographically. During the course of these studies,
we discovered that isolated samples of rac-5/meso-5 (with a 2 to 1
ratio, in tetrahydrofuran-d8 solvent) slowly isomerized to enhance
the relative amount of rac isomer in solution. Similar behavior was
observed for isolated samples of rac-6/meso-6 (with a 1 to 3
ratio, in tetrahydrofuran-d8 solvent); the proportion of the rac
isomer increased over time. We monitored the kinetics of these isomerizations
using 31P{1H} NMR spectroscopy, and found that the
isomerization rate for 5 is more than 15 fold greater than that for 6.
In separate experiments, we found that these rac/meso equilibrium ratios
are reached at a faster rate when isolated samples of 5 and 6 are
exposed to tetrabutylammonium chloride in tetrahydrofuran-d8
solvent. Addition of neutral ligand, 3, to isolated samples of 5
and 6 in tetrahydrofuran-d8 solvent also accelerates
isomerization, but not as dramatically as tetrabutylammonium chloride. We
invoke nucleophile (either chloride or the phosphine interannular linker)
promoted dissociation of one cyclopentadienyl ring as the critical mechanistic
step in our observed rac/meso isomerizations. In future work, we will
capitalize on our ability to affect the controlled isomerization of
rac-5/meso-5 and rac-6/meso-6 and will use pure rac
samples for conversion to the zirconocene dichloride olefin polymerization
precatalyst, rac-{PhP(3-t-Bu-C5H3)2}ZrCl2.
During year
2, the chemistry for project 2 was advanced to allow for submission of a
manuscript that is currently under review. We developed a stepwise synthetic
method for assembly of a bis(imino)pyridine ligand with t-butyl substituents on
the imino carbon atoms, 2,6-{(2,4-Me2-C6H3)NC(t-Bu)}2C5H3N
(10). The use of an imidoyl chloride (8) as an electrophile in
combination with in situ generated 2-bromo-6-lithiopyridine has not been
reported prior to this work. We were able to characterize mono(imino)pyridine,
9, and bis(imino)pyridine 10, via single crystal X-ray
diffraction. Compound 10 adopts an interesting “closed” geometry, which
is quite different from the more “open” geometry reported in the literature for
2,6-{(2,4-Me2-C6H3)NC(Me)}2C5H3N,
the analogous ligand with methyl substituents on the imino carbon atoms. We
hypothesize that the thermodynamic preference for the “closed” geometry of our
ligand (both in the solid state and also borne out by gas-phase calculations)
hinders metallation using iron(II)chloride. Indeed, metallation attempts of
10 under forcing conditions (such as use of refluxing 1-hexanol over the
course of days) does not yield the desired iron(II)chloride complex, 11.
In future work, we plan to use our new stepwise synthetic method to prepare C1-symmetric
ligands with differing substitution on the imino carbon atoms; metallation will
be attempted using iron(II)chloride as well as other iron(II) sources, such as
FeCl2(THF)1.5.
The zirconium
chemistry described above (project 1) has been accepted as a full paper in press
for the Journal of Organometallic Chemistry. Notably, Jonathan Axtell is the
first author for this publication; he is currently a junior undergraduate
student and was supported for years 1 and 2 of this grant. We have another full
paper under review for Dalton Transactions; this paper recounts the bis
imino(pyridine) chemistry described above (project 2). This submission also has
an undergraduate first author, Janelle Steves; she is a current junior and was
supported for years 1 and 2 of the grant. In order to further disseminate the
work carried out during the grant period, we intend to submit abstracts for two
presentations at the ACS National Meeting in Spring 2009.