
Back to Table of Contents
44832-AC4
ortho-Bis(methylium)phenylene and Related Dications: Synthesis, Characterization, and Anion Complexation
François P. Gabbaï, Texas A&M University
In this funding period, the funds
provided by this PRF grant have been used to support our research in three
distinct areas. The results will
therefore be presented in three separate sections.
1) Borenium and
boronium cations – Development of a turn-on fluoride indicator
Hoping to discover new methods to sense anions,
we have investigated the synthesis of novel cationic boron compounds including the
borenium cations [1]+ and
the boronium cation [2-DMAP]+
(DMAP = p-dimethylaminopyridine). These compounds have been prepared from the
corresponding fluoride derivatives by treatment with trimethylsilyl triflate
and DMAP. In
the presence of iodide ions, [2–DMAP]+
behaves as a turn–on fluoride indicator and reacts with fluoride ions to afford
the corresponding brightly fluorescent difluoride 2–F. This fluorescence
increase results from the greater sensitivity of cationic [2–DMAP]+ (when compared to neutral 2–F) to the external heavy atom effects imparted by I-.

2) Polyfunctional
Lewis acidic boranes as receptors for fluoride and cyanide anions
We have synthesized a
series of novel naphthalene-based multidentate boranes and evaluated their
affinity for fluoride anions. In the
course of these studies, we have discovered that the trinuclear Lewis acid 3 is able to bind two fluoride
anions. However, binding of the second
fluoride anion is much less favorable than that of the first because of
unfavorable Coulombic and steric effects.
Comparative studies indicates that the fluoride binding constant of 3 is similar to that of simple B/Hg
bidentate Lewis acids such as 4 but
significantly lower than that of bidentate diboranes such as 5.

In an effort to strengthen
the host guest interaction, we have more recently considered cationic
multidentate boranes such as [o-6]+. Remarkably, the reaction of equimolar amounts
of [o-6]I and p-6-F in CDCl3 leads to [p-6]+
and p-6-F in a quantitative yield, indicating that the fluoride affinity
of [o-6]+ is far superior than
that of [p-6]+. More
quantitative information could be gained from fluoride titration experiments
carried out in MeOH. In this solvent,
the fluoride binding constant of [o-6]+ (K > 106 M-1)
exceeds the measurable range and is at least 4 orders of magnitude higher than
that measured for [p-6]+ (K = 400 (±50) M-1). Clues to the higher fluoride affinity of [o-6]+
were derived from crystallographic measurements which pointed to the presence
of a bonding interaction between the fluorine and phosphorus atoms of o-6-F. The crystal structure of this derivative
confirmed this proposal. Specifically,
the boron bound fluorine atom F(1) (B(1)-F(1) = 1.482(3) Å) is located
only 2.666(2) Å away from the P(1) atom, which is well within the sum of the
van der Waals radii of the two elements (ca. 3.45 Å) (Figure 1). Another conspicuous feature concerns the
F(1)-P(1)-C(31) angle of 176.36(9)°.

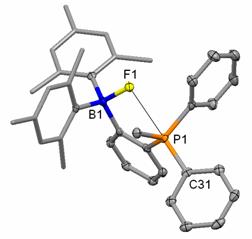
Figure 1: Crystal structure of o-6-F
These
results indicate the presence of a B-F→P interaction, which
contributes to the increased fluorophilicity of [o-6]+. While the F→P interaction must bear a
large electrostatic component, an Atoms-in-Molecules (AIM) analysis carried out
at the DFT optimized geometry indicates the presence of a bond path connecting
the two atoms (Figure 2). Furthermore,
an Natural Bond Orbital (NBO) analysis identifies a donor-acceptor
interaction involving a fluorine lone-pair as a donor and the
phosphorus-carbon σ*-orbital as the acceptor. Thus, [o-6]+ can be regarded as a
cationic bidentate Lewis acid, whose high fluoride affinity arises from both
fluoride ion chelation and Coulombic attractions. In turn, these results further demonstrate
that Coulombic and chelate effects are additive and can be combined to boost
the anion affinity of Lewis acidic hosts.
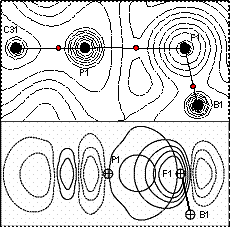
Figure 2: AIM and NBO analyses of
the B-F→P interaction in o-6-F. Top:
AIM electron density map with relevant bond paths and bond critical points.
Bottom: NBO contour plot showing the lp(F)→σ*(P-C)
interaction.3) Unusual
agostic interactions
We have synthesized the cationic
fluorosilane 1-(dimethylfluorosilane)-8-(9-xanthylium)naphthalenediyl ([7]+) as a tetrafluoroborate
salt and converted it into
1-(dimethylfluorosilane)-8-(9H-xanthene)naphthalenediyl (8) by reaction with NaBH4. The most interesting aspect of this chemistry
pertains to the presence of an agostic C-H → Si interaction in 8. This interaction, which is
characterized by a Si-H separation of 2.32(2) Å and a F-Si-H
angle of 177.0(5)°, leads the silicon atom to adopt a distorted
trigonal-bipyramidal geometry.

Back to top







