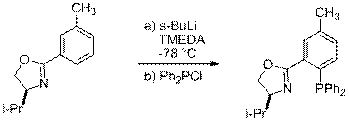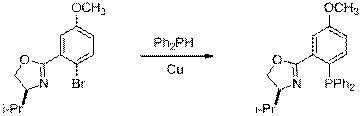46722-B4
Hammett Studies of P,N-Chiral Ligands
Summary
The majority of the work centered on synthesizing (preparing) new chiral ligands (and ligand precursors) for eventual study. Despite much time and effort we were not able to generalize the Pd or Ni catalyzed cross-coupling route to 5'-substituted ligands. We have had success with a newly reported Cu-catalyzed coupling reaction preparing one new ligand using the “Buchwald-Stoltz” conditions for aryl bromides. Very recently we have extended this protocol to aryl triflates, substrates not reported in the literature, but much more available by our current protocols. We stand poised to exploit this reaction, synthesize the remaining ligands we are after, and then begin our Hammett studies.
I. 4'-Ligand Precursors
Starting from commercial materials were were able to synthesize the following ligand precursors (Eq 1-4, Scheme 1-2).
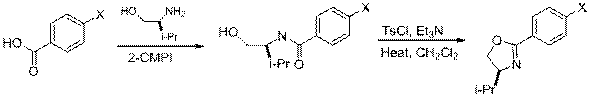
A. Nitro (-NO2) and Trifluoromethyl (-CF3)
| Eq 1 |
1a,b X = NO2, CF3 2a,b 3a,b |
|
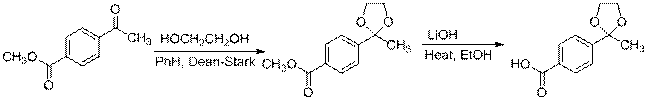
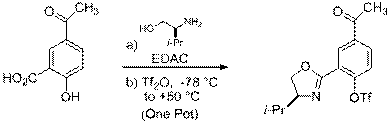
B. Acetyl (-COCH3)
|
4 5 6
|
|
6 7 8
|
Scheme 1 Route to Protected 4'-Acetyl Ligand Precursor
|
II. 5'-Ligand Precursors
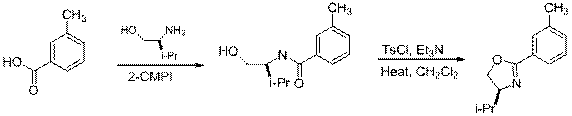
A. Methyl (CH3)
| Eq 2 |
9 10 11
|
|
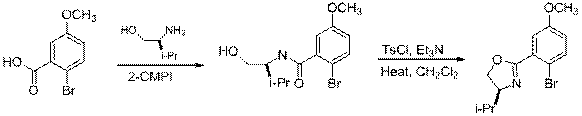
B. Acetyl (-COCH3)
| Eq 3 |
12 13
|
|
C. Methoxy (-OCH3)
| Eq 4 |
14 15 16
|
|
III. 4'-Ligand Synthesis
A. Acetyl (-COCH3)
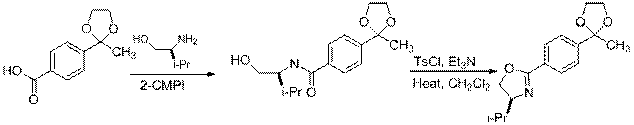
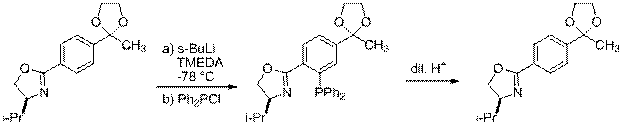
Evan Smith worked on the ortho-lithiation of 8 to produce ligand 17 (scheme 2). Ultimately we plan to deprotect the ketal to form ligand 18 as well. Evan's preliminary work shows that this deprotection is facile.
|
8 17 18
|
Scheme 2 Route to Protected 4'-Acetyl Ligand
|
IV. 5'-Ligand Synthesis
A Methyl (-CH3)
Zach DeVore worked to prepare ligand 19 (Eq 5). Zach was only partially successful in this work. He obtained promising evidence for the ligand. I was able to follow up on his work and synthesize and fully characterize ligand 19.
| Eq 5 |
11 19
|
|
B. Model Study with Acetyl (-COCH3)
Because of our keen interest in the aryl triflate route, we prepared model compound 21 for exploratory study of different reaction conditions (scheme 3). Bases on some literature precedents we were able obtain phosphine 22 under several reaction conditions. Palladium or Ni catalysis with Ph2P-TMS as well as Ni catalysis with Ph2P-Cl and stoichiometric Zn all worked well with the model system. The Zn was key for several reasons including the prevention of the formation of phosphine oxide. This oxidation of phosphorus was ultimately was caused Jay Fitzgerald much trouble we believe.
|
20 21 22
|
Scheme 3 Model System Route to Pd and Ni Cross-Coupling
|
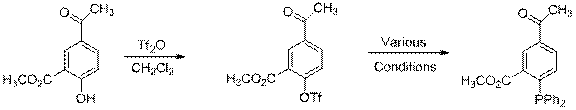
C. Acetyl (-COCH3) via Cu
Despite our success with the model system (21 to 22), under no set or combination of previously successful conditions were we able to synthesize ligand 23 (Eq 6). This consumed a great deal of time and effort. We now have some success with the Buchwald-Stoltz Cu(I) catalyst system and have produced 23 in 30% unoptimized yield. Aryl triflates have not previously been reported as substrates for this reaction.
| Eq 6 |
13 23
|
|
D . Methoxy (-OCH3) via Cu
The same conditions worked as described in the literature starting with aryl bromides to prepare 5'-methoxy ligand 24 (Eq 7). Note that this is one of the substituents that we were unable to obtain previously via the aryl triflate and Pd or Ni catalysis. Thus, we are extremely optimistic about the utility of aryl bromides with this new catalyst system. We have obtained several other aryl bromides for analogous reactions to produce these elusive 5'-substituted ligands.
| Eq 7 |
16 24
|
|
V. Conclusions and Future Directions
We were able to synthesize three new ligands, as well as numerous precursor molecules. We did not succeed in generalizing the Pd or Ni-catalyzed cross-coupling of the aryl triflates, but the Cu-catalyzed coupling of aryl bromides and triflates should provide new access to these ligands.. We will then continue with our Hammett studies of these new ligands.