

44942-AC3
Characterization of Catalytic Imide Group Transfer Reactions with Iron Catalysts
Narrative Report (September 2008)
The report has subsections corresponding to the three main goals of the proposal, and a fourth section addresses unforeseen results gained under PRF support.
(1) Characterization of Iron(III) Imido Complexes. The imido complex we have reported is shown at the left of Figure 1, but it is too unstable for crystallography and is formed in less than 70% yield. One of our aims is to create more stable imido intermediates for crystallography and/or EXAFS analysis (Extended X-ray Absorption Fine Structure, which shows metal-ligand bond distances). Figure 1 shows two ligand modifications that we have made. First, adding tert-butyl groups to the backbone of the ligand adds steric hindrance (Figure 1, center). Using this ligand, we have generated solutions that contain 85-90% of the metastable imido species, as judged by 1H NMR spectroscopy. We have obtained EXAFS data (in collaboration with Serena DeBeer-George at Stanford Synchrotron Laboratory), which show a 1.69 distance to a light-atom scatterer that has 50-70% occupancy. This short distance is indicative of the postulated Fe=N double bond, and with the Fe=N distances in DFT computations by our collaborator Thomas Cundari (University of North Texas). In fall 2008, a student will travel to Germany to collect Mossbauer spectra of freshly prepared samples, in collaboration with Eckhard Bill, Max-Planck-Institut, Mulheim, Germany.
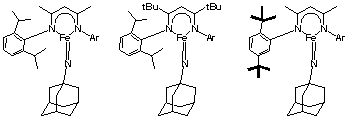
Another ligand modification is shown at the right of Figure 1. This ligand has no weak C-H bonds near the metal site, because the ortho-isopropyl groups are replaced by tert-butyl groups. In an exciting development, an iron(I) complex of this ligand reacts with azide to form a long-lived (days at room temperature) species with 1H NMR spectra similar to the imido complexes of other ligands (which last only a few hours at room temperature). These results are most consistent with formation of the desired imido complex, and efforts to crystallize this complex for X-ray diffraction analysis are underway.
(2) Hydrogen Atom Abstraction and Amination Reactions. This section of the proposed work is dependent on part (1), in that it requires a ligand that is not attacked by the imido group. The promising preliminary results on the long-lived imido complex strongly suggest that we will be able to address intramolecular H-atom abstractions soon.
(3) Catalytic Carbodiimide and Aziridine Formation through Group Transfer. We are writing up our mechanistic studies on the catalytic formation of tBuNCNAd from adamantyl azide (AdN3) and t-butyl isocyanide (tBuNC), which was described in detail in the 2007 report. Interestingly, catalytic group transfer also occurs with CO to form AdNCO, but the reaction with phosphines to give R3P=NAd does not turn over. Attempts to observe aziridination have not yet been successful, but ligand modifications (described above) are hoped to give promising results.
(4) Other Results. Last year's report described preliminary information on two interesting species: a tetrazene complex LMeFe(AdNNNNAd), and a "hexazene" complex (LMeFe)2(AdNNNNNNAd). Each of these contains a very interesting polynitrogen ligand, and each has been evaluated in detail.
The "hexazene" group (AdNNNNNNAd) is previously unknown in transition-metal chemistry. We characterized the first two hexazene complexes, and determined the oxidation state of the metal and ligand using Mossbauer spectroscopy and magnetic susceptibility (in collaboration with Eckhard Bill). This work was published as a Communication in J. Am. Chem. Soc., and was even covered as a Science Concentrate in Chemical and Engineering News (April 28, 2008).
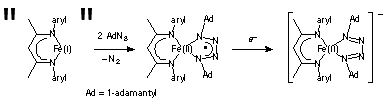
The tetrazene complex LMeFe(AdNNNNAd) is interesting because it could be formulated as an iron(I) complex with a neutral ligand, as an iron(II) complex with a radical anion ligand, or an iron(III) complex with a dianionic ligand (Figure 2). Mssbauer spectra and DFT calculations have definitively shown the iron(II)-radical ligand resonance form is most accurate. Interestingly, reduction by one electron gives [LMeFe(AdNNNNAd)]–, in which the ligand, rather than the metal, has been reduced. Although spectroscopic and theoretical studies are ambiguous on the electron distribution in this compound, the crystal structure shows clear signs of ligand reduction. This first detailed study on tetrazene redox chemistry is also complete, and is being submitted to Angew. Chem. for publication.